A dead-end street of protein folding
... Amino acid sequences of globular proteins encode their 3D-structures linked to their biological function. More evidence supports that for many proteins a second, well organized, but quite different 3Dstructure also exists. The latter types of conformers have an architecture similar to the aggregated ...
... Amino acid sequences of globular proteins encode their 3D-structures linked to their biological function. More evidence supports that for many proteins a second, well organized, but quite different 3Dstructure also exists. The latter types of conformers have an architecture similar to the aggregated ...
Supplementary data 1,2,3,4,6,7,8,9 include N, Total (ProtScore)
... Supplementary data 1,2,3,4,6,7,8,9 include N, Total (ProtScore), %Cov(95), Accession, Name, Conf, Sequence,Modifications for the identified proteins. The definitions of the table fields are described as follows: N is the rank of the specified protein relative to all other proteins in the list of det ...
... Supplementary data 1,2,3,4,6,7,8,9 include N, Total (ProtScore), %Cov(95), Accession, Name, Conf, Sequence,Modifications for the identified proteins. The definitions of the table fields are described as follows: N is the rank of the specified protein relative to all other proteins in the list of det ...
Key Points Folding
... Prions and Protein Folding • Protein structure (primary, secondary, tertiary) • Proteins have many possible conformations (ways to fold up into a 3D structure) • Proteins can spontaneously fold into the correct (biologically functional) 3D structure demonstrated by Christian Anfinsen in the 1950’s • ...
... Prions and Protein Folding • Protein structure (primary, secondary, tertiary) • Proteins have many possible conformations (ways to fold up into a 3D structure) • Proteins can spontaneously fold into the correct (biologically functional) 3D structure demonstrated by Christian Anfinsen in the 1950’s • ...
Project description
... proteins are the participants of the termination stage. For instance, besides two “canonical” and the well-known termination factors eRF1 and eRF3, in humans we have two others interesting proteins important for termination, Dbp5 and PABP. These proteins have a wide range of activities in the cells, ...
... proteins are the participants of the termination stage. For instance, besides two “canonical” and the well-known termination factors eRF1 and eRF3, in humans we have two others interesting proteins important for termination, Dbp5 and PABP. These proteins have a wide range of activities in the cells, ...
CH 107 SI Summer 2015 Worksheet 13 Answers What are the two
... 1. What are the two major types of secondary protein structure and what bonds are present in each? α-helices and β-sheets Hydrogen bonds 2. What types of interactions can be present in tertiary protein structure? Rank the interactions from strongest to weakest. disulfide bonds >> salt bridges > hydr ...
... 1. What are the two major types of secondary protein structure and what bonds are present in each? α-helices and β-sheets Hydrogen bonds 2. What types of interactions can be present in tertiary protein structure? Rank the interactions from strongest to weakest. disulfide bonds >> salt bridges > hydr ...
Research Interests
... (Pvu II), mammalian (DNMT1, DNMT3) and bacterial ( BseCI) DNA methyltransferases and of RNA-binding proteins (Rop) have been performed. Applications resulting from this work include the engineering of "programmable" endonucleases and novel DNA specificities for gene therapy and endonucleases which a ...
... (Pvu II), mammalian (DNMT1, DNMT3) and bacterial ( BseCI) DNA methyltransferases and of RNA-binding proteins (Rop) have been performed. Applications resulting from this work include the engineering of "programmable" endonucleases and novel DNA specificities for gene therapy and endonucleases which a ...
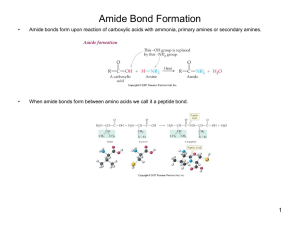
Amide Bond Formation
... Amide bonds form upon reaction of carboxylic acids with ammonia, primary amines or secondary amines. ...
... Amide bonds form upon reaction of carboxylic acids with ammonia, primary amines or secondary amines. ...
Abstract
... protein 3D structure, interactions and recognition in signaling networks. Modern sequencing technologies provide us with a rich source of data about the evolutionary history of proteins. Inferring a joint probability distribution of amino acid sequences that are members of a protein family, signals ...
... protein 3D structure, interactions and recognition in signaling networks. Modern sequencing technologies provide us with a rich source of data about the evolutionary history of proteins. Inferring a joint probability distribution of amino acid sequences that are members of a protein family, signals ...
Life’s molecular diversity is based on the properties of carbon 8/25/2011 1
... fibers that make up connective tissues such as tendons and ligaments. • Contractile proteins: Found in muscles • Defensive proteins: The antibodies of the ...
... fibers that make up connective tissues such as tendons and ligaments. • Contractile proteins: Found in muscles • Defensive proteins: The antibodies of the ...
Amino acids
... solubility (scleroproteins, albumins, histones, globulins) function (proteins of basic metabolism, specialized cells) ...
... solubility (scleroproteins, albumins, histones, globulins) function (proteins of basic metabolism, specialized cells) ...
ppt - Scientific Data Analysis Lab
... Disordered regions (DRs) are entire proteins or regions of proteins which lack a fixed tertiary structure, essentially being partially or fully unfolded. Such disordered regions have been shown to be involved in a variety of functions, including DNA recognition, modulation of specificity/affinity of ...
... Disordered regions (DRs) are entire proteins or regions of proteins which lack a fixed tertiary structure, essentially being partially or fully unfolded. Such disordered regions have been shown to be involved in a variety of functions, including DNA recognition, modulation of specificity/affinity of ...
Knuffke Prezi- Macromolecules
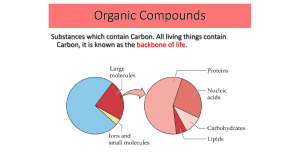
... Organic Compounds Substances which contain Carbon. All living things contain Carbon, it is known as the backbone of life. ...
... Organic Compounds Substances which contain Carbon. All living things contain Carbon, it is known as the backbone of life. ...
1.Contrast and compare the structure of a saturated fat versus an
... 1. Contrast and compare the structure of a saturated fat versus an unsaturated fat. 2. Identify and describe the four levels of protein structure. 3. Speculate (predict) on why a change in pH or Na+ concentration could cause a protein to lose its secondary or tertiary structure and denature. 4. Disc ...
... 1. Contrast and compare the structure of a saturated fat versus an unsaturated fat. 2. Identify and describe the four levels of protein structure. 3. Speculate (predict) on why a change in pH or Na+ concentration could cause a protein to lose its secondary or tertiary structure and denature. 4. Disc ...
Ch 4 Reading Guide
... ______________________ residues. 12. How are hydrogen bonds maintained in the interior of a globular protein? 13. Define motif (super-secondary structure.) 14. Define domain. 15. Quaternary structure refers to the arrangement of _______________ and the nature of their interactions. 16. Explain the e ...
... ______________________ residues. 12. How are hydrogen bonds maintained in the interior of a globular protein? 13. Define motif (super-secondary structure.) 14. Define domain. 15. Quaternary structure refers to the arrangement of _______________ and the nature of their interactions. 16. Explain the e ...
Proteins and Nucleic Acids Proteins (pp.46-48) Monomer
... Outline of Information to pull out of pp. 46-50 in Text book ...
... Outline of Information to pull out of pp. 46-50 in Text book ...
Biochem-5012.3B - Center for Structural Biology
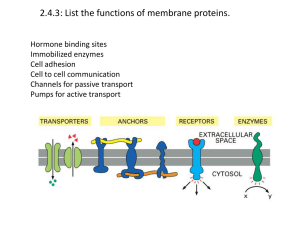
... Factors Bound by Different Protein Classes • Transport- O2/CO2, cholesterol, metals, sugars • Storage- metals, amino acids, • Immune response- foreign matter (antigens) • Receptors- regulatory proteins, transmitters • Structure- other structural proteins ...
... Factors Bound by Different Protein Classes • Transport- O2/CO2, cholesterol, metals, sugars • Storage- metals, amino acids, • Immune response- foreign matter (antigens) • Receptors- regulatory proteins, transmitters • Structure- other structural proteins ...
Proteins - Wesleyan College Faculty
... http://learn.genetics.utah.edu/content/begin/dna/transcribe/ ...
... http://learn.genetics.utah.edu/content/begin/dna/transcribe/ ...
Intrinsically disordered proteins

An intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure. IDPs cover a spectrum of states from fully unstructured to partially structured and include random coils, (pre-)molten globules, and large multi-domain proteins connected by flexible linkers. They constitute one of the main types of protein (alongside globular, fibrous and membrane proteins).The discovery of IDPs has challenged the traditional protein structure paradigm, that protein function depends on a fixed three-dimensional structure. This dogma has been challenged over the last decades by increasing evidence from various branches of structural biology, suggesting that protein dynamics may be highly relevant for such systems. Despite their lack of stable structure, IDPs are a very large and functionally important class of proteins. In some cases, IDPs can adopt a fixed three-dimensional structure after binding to other macromolecules.