AP Chemistry Ch. 3 Sections 3.7-3.8 Notes Chemical Equations
... For example, when hydrochloric acid in aqueous solution is added to solid sodium hydrogen carbonate, the products carbon dioxide gas, liquid water, and sodium chloride (which dissolves in the water) are formed: HCl (aq) + NaHCO3 (s) → CO2 (g) + H2O (l) + NaCl (aq) The relative numbers of reactants a ...
... For example, when hydrochloric acid in aqueous solution is added to solid sodium hydrogen carbonate, the products carbon dioxide gas, liquid water, and sodium chloride (which dissolves in the water) are formed: HCl (aq) + NaHCO3 (s) → CO2 (g) + H2O (l) + NaCl (aq) The relative numbers of reactants a ...
Balancing Equations
... Example: Sodium + Chlorine Sodium chloride 2. Chemical equations—Show the formulas of reactants and products. Example: Na + Cl2 NaCl (Not Balanced Yet!) 3. Skeleton equations—Equations that are not yet balanced to represent what actually occurs in the real world (see example above) ...
... Example: Sodium + Chlorine Sodium chloride 2. Chemical equations—Show the formulas of reactants and products. Example: Na + Cl2 NaCl (Not Balanced Yet!) 3. Skeleton equations—Equations that are not yet balanced to represent what actually occurs in the real world (see example above) ...
SCIENCE 9
... has its own distinct properties and cannot be broken down into simpler substances by means of a chemical change. COMPOUNDS- are pure substances that are made up of two or more elements chemically combined together. Compounds can be broken down into elements again by chemical means ...
... has its own distinct properties and cannot be broken down into simpler substances by means of a chemical change. COMPOUNDS- are pure substances that are made up of two or more elements chemically combined together. Compounds can be broken down into elements again by chemical means ...
physical and chemical change
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
physical and chemical change
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
New Standard Chemical Resistance PDF
... FLOORING CHEMICAL RESISTANCE Beaulieu’s® New Standard™ flooring is resistant to a variety of chemicals and is tested under the ASTM F925 for resistance to surface deterioration when exposed to various chemical reagents. Chemicals that can be found in the home, industrial or medical facilities that a ...
... FLOORING CHEMICAL RESISTANCE Beaulieu’s® New Standard™ flooring is resistant to a variety of chemicals and is tested under the ASTM F925 for resistance to surface deterioration when exposed to various chemical reagents. Chemicals that can be found in the home, industrial or medical facilities that a ...
Section B - 8 UNO NON-WASTE CHEMICAL STORAGE
... Based on the information given in the EPA document mentioned above, eight general compatibility categories have been developed for use at UNO. Incompatibilities within those categories are broken down into classes. These compatibility classes are described below. The compatibility classes are prior ...
... Based on the information given in the EPA document mentioned above, eight general compatibility categories have been developed for use at UNO. Incompatibilities within those categories are broken down into classes. These compatibility classes are described below. The compatibility classes are prior ...
1-BUTANESULFONIC ACID SODIUM SALT
... Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical attention immediately. Skin: In case of contact, flush skin with plenty of water for at least 15 minutes while removing contaminated clothes and shoes. Get medical ...
... Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical attention immediately. Skin: In case of contact, flush skin with plenty of water for at least 15 minutes while removing contaminated clothes and shoes. Get medical ...
1st mid unit test formative (pre-test)
... The horizontal rows of the periodic table are called periods. The horizontal columns are called families or groups. Metals are on the left and in the center of the table. Non-metals are located on the right-hand side of the table. Metals are separated from non-metals by a staircase of elements calle ...
... The horizontal rows of the periodic table are called periods. The horizontal columns are called families or groups. Metals are on the left and in the center of the table. Non-metals are located on the right-hand side of the table. Metals are separated from non-metals by a staircase of elements calle ...
1st mid unit test formative (pre-test)
... The horizontal rows of the periodic table are called periods. The horizontal columns are called families or groups. Metals are on the left and in the center of the table. Non-metals are located on the right-hand side of the table. Metals are separated from non-metals by a staircase of elements calle ...
... The horizontal rows of the periodic table are called periods. The horizontal columns are called families or groups. Metals are on the left and in the center of the table. Non-metals are located on the right-hand side of the table. Metals are separated from non-metals by a staircase of elements calle ...
PowerPoint for Cornell Notes
... or a bee? Bee stings are acidic in nature, which is why a household remedy for a bee sting is baking soda or sodium bicarbonate, which is a basic substance. A wasp sting, on the other hand, is mildly basic, so a household remedy for this will be vinegar, also known as acetic acid. These simple treat ...
... or a bee? Bee stings are acidic in nature, which is why a household remedy for a bee sting is baking soda or sodium bicarbonate, which is a basic substance. A wasp sting, on the other hand, is mildly basic, so a household remedy for this will be vinegar, also known as acetic acid. These simple treat ...
Matter, Mass and Weight
... Elements are substances that cannot be decomposed into simpler substances by physical or chemical means. Like all pure substances, it has its own set of physical and chemical properties. At room temperature, 75 elements are solids, 11 are gases, 2 (mercury and bromine) are liquids. Each element is r ...
... Elements are substances that cannot be decomposed into simpler substances by physical or chemical means. Like all pure substances, it has its own set of physical and chemical properties. At room temperature, 75 elements are solids, 11 are gases, 2 (mercury and bromine) are liquids. Each element is r ...
Chemistry 2011-2012
... SC6a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. SC6b. Collect data and calculate the amount of heat given off or taken in by chemical or physical processes. SC6c. Analyzing (both conceptually and quantitatively) flow of energy during change of state (phase) ...
... SC6a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. SC6b. Collect data and calculate the amount of heat given off or taken in by chemical or physical processes. SC6c. Analyzing (both conceptually and quantitatively) flow of energy during change of state (phase) ...
Chemistry Test Review - Greenslime Home Page
... 6. How many protons are in one molecule of NH4? Two molecules? a. 11 protons in one molecule b. 22 protons in 2 molecules 7. Describe the chemical formula: 4NaHCO3 a. 4 molecules of a compound containing 1 atom of the element Sodium, 1 atom of the element Hydrogen, 1 atom of the element Carbon and 3 ...
... 6. How many protons are in one molecule of NH4? Two molecules? a. 11 protons in one molecule b. 22 protons in 2 molecules 7. Describe the chemical formula: 4NaHCO3 a. 4 molecules of a compound containing 1 atom of the element Sodium, 1 atom of the element Hydrogen, 1 atom of the element Carbon and 3 ...
ap chemistry – 2013-2014
... AP CHEMISTRY – 2013-2014 Course Description: This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. This course is structured around six big ideas that include: Structure of matter, properties of matter-characteristic ...
... AP CHEMISTRY – 2013-2014 Course Description: This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. This course is structured around six big ideas that include: Structure of matter, properties of matter-characteristic ...
Modified Osteo-Odonto Keratoprosthesis (MOOKP) in patients
... Post-operative Snellen BCVA ranged from 0.4.to 0.7 except for three eyes. ...
... Post-operative Snellen BCVA ranged from 0.4.to 0.7 except for three eyes. ...
Chapter #3
... There are N of the above equations, one for each element (atom type) in the reaction. Generally there are M coefficients to find using the N equations. Unfortunately, in most chemical equations, M > N. Usually, we have the case that M = N+1. Thus, we need to find one additional equation. One simple ...
... There are N of the above equations, one for each element (atom type) in the reaction. Generally there are M coefficients to find using the N equations. Unfortunately, in most chemical equations, M > N. Usually, we have the case that M = N+1. Thus, we need to find one additional equation. One simple ...
Chapter 1
... *Notes-The substance that forms in a chemical reaction is called a _____Product__________. You produce a product in a chemical reaction. C. The Importance of Accuracy D. The Reason Equations Must be Balanced *Notes-The Law of Conservation of Mass dictates that chemical equations must be balanced bec ...
... *Notes-The substance that forms in a chemical reaction is called a _____Product__________. You produce a product in a chemical reaction. C. The Importance of Accuracy D. The Reason Equations Must be Balanced *Notes-The Law of Conservation of Mass dictates that chemical equations must be balanced bec ...
Chapter 2 Introduction to Chemistry
... A substance is a particular kind of matter that has a uniform and definite composition. Pure substances contain only one kind of matter ...
... A substance is a particular kind of matter that has a uniform and definite composition. Pure substances contain only one kind of matter ...
Chemical Reactions
... • Uses chemical formulas and symbols to describe a chemical reaction and the product it produces (see below) Symbol ...
... • Uses chemical formulas and symbols to describe a chemical reaction and the product it produces (see below) Symbol ...
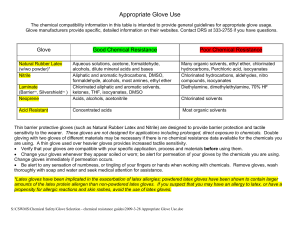
Appropriate Glove Use
... alcohols, dilute mineral acids and bases Aliphatic and aromatic hydrocarbons, DMSO, ...
... alcohols, dilute mineral acids and bases Aliphatic and aromatic hydrocarbons, DMSO, ...
Chemical industry

The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials (oil, natural gas, air, water, metals, and minerals) into more than 70,000 different products.