wahideh chemistry eportfolio hw
... strongly to other elements. Its compounds are very difficult to break apart. It was not until 1807 ...
... strongly to other elements. Its compounds are very difficult to break apart. It was not until 1807 ...
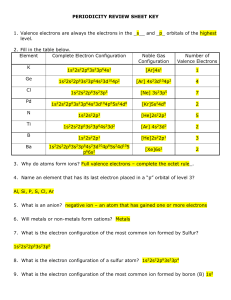
periodicity review sheet key
... __D___ 16. Going down a group of elements, the ionization energy? __D___ 17. Going left to right across a period, the atomic radius? __R___ 18. Going down a group 2, then number of valence electrons? __D___ 19. As a metallic atom becomes a cation, its radius? __D___ 20. As the atomic radius of the n ...
... __D___ 16. Going down a group of elements, the ionization energy? __D___ 17. Going left to right across a period, the atomic radius? __R___ 18. Going down a group 2, then number of valence electrons? __D___ 19. As a metallic atom becomes a cation, its radius? __D___ 20. As the atomic radius of the n ...
Periodic Table2011
... • The nitrogen family is named after the element that makes up 78% of our atmosphere. • This family includes nonmetals, metalloids, and metals. • Atoms in the nitrogen family have 5 valence electrons. They tend to share electrons when they bond. • Other elements in this family are phosphorus, arseni ...
... • The nitrogen family is named after the element that makes up 78% of our atmosphere. • This family includes nonmetals, metalloids, and metals. • Atoms in the nitrogen family have 5 valence electrons. They tend to share electrons when they bond. • Other elements in this family are phosphorus, arseni ...
Unit 3 Periodic Table Vocabulary
... Alkali Metals - Any of a group of soft, white, low-density, low-melting, highly reactive metallic elements, including lithium, sodium, potassium, rubidium, cesium, and francium. Sentence: You can find alkali metals in the periodic table. ...
... Alkali Metals - Any of a group of soft, white, low-density, low-melting, highly reactive metallic elements, including lithium, sodium, potassium, rubidium, cesium, and francium. Sentence: You can find alkali metals in the periodic table. ...
Chapter 11 Chemical Reactions
... Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a ...
... Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a ...
Chapter 6 Review“The Periodic Table”
... 1. How is the number of neutrons in the nucleus of an atom calculated? Mass – atomic number 2. All atoms are neutral, with the number of protons equaling the ___. # electrons 3. Isotopes of the same element have different _____. # neutrons 4. Using the periodic table, determine the number of neutron ...
... 1. How is the number of neutrons in the nucleus of an atom calculated? Mass – atomic number 2. All atoms are neutral, with the number of protons equaling the ___. # electrons 3. Isotopes of the same element have different _____. # neutrons 4. Using the periodic table, determine the number of neutron ...
Chem Sheets to Memorize SOLUBILITY CHART
... *You will need to be able to write molecular chemical reactions and do mole conversions to do these questions. 1. 30.5 g of sodium metal reacts with a solution of excess lithium bromide. How many grams of lithium metal are produced? 2. How many molecules are in 100. L of potassium hydroxide solution ...
... *You will need to be able to write molecular chemical reactions and do mole conversions to do these questions. 1. 30.5 g of sodium metal reacts with a solution of excess lithium bromide. How many grams of lithium metal are produced? 2. How many molecules are in 100. L of potassium hydroxide solution ...
Chem Sheets to Memorize
... *You will need to be able to write molecular chemical reactions and do mole conversions to do these questions. 1. 30.5 g of sodium metal reacts with a solution of excess lithium bromide. How many grams of lithium metal are produced? 2. How many molecules are in 100. L of potassium hydroxide solution ...
... *You will need to be able to write molecular chemical reactions and do mole conversions to do these questions. 1. 30.5 g of sodium metal reacts with a solution of excess lithium bromide. How many grams of lithium metal are produced? 2. How many molecules are in 100. L of potassium hydroxide solution ...
Chem Sheets to Memorize
... *You will need to be able to write molecular chemical reactions and do mole conversions to do these questions. 1. 30.5 g of sodium metal reacts with a solution of excess lithium bromide. How many grams of lithium metal are produced? 2. How many molecules are in 100. L of potassium hydroxide solution ...
... *You will need to be able to write molecular chemical reactions and do mole conversions to do these questions. 1. 30.5 g of sodium metal reacts with a solution of excess lithium bromide. How many grams of lithium metal are produced? 2. How many molecules are in 100. L of potassium hydroxide solution ...
7A The Periodic Table
... Every element is given a symbol of one or two letters. For example, the symbol for hydrogen is a capital letter H. The symbol for lithium is two letters, Li. Each element also has a unique number called the atomic number. The atomic number is the number of protons in the nucleus of all atoms of that ...
... Every element is given a symbol of one or two letters. For example, the symbol for hydrogen is a capital letter H. The symbol for lithium is two letters, Li. Each element also has a unique number called the atomic number. The atomic number is the number of protons in the nucleus of all atoms of that ...
Chemical Reactions and Stoichiometry
... Chapter 8 Types of Chemical Reactions VII. Types of Chemical Reactions - Doing reactions in a lab can be dangerous, time consuming and/or expensive. Recognizing patterns allows scientists to predict the products of many reactions. a. Synthesis – also called combination reactions. Two or more reactan ...
... Chapter 8 Types of Chemical Reactions VII. Types of Chemical Reactions - Doing reactions in a lab can be dangerous, time consuming and/or expensive. Recognizing patterns allows scientists to predict the products of many reactions. a. Synthesis – also called combination reactions. Two or more reactan ...
Name: (1 of 2) Math Set # 13 Protons,
... The number of protons is ALWAYS the same for an atom of a specific element. Germanium ALWAYS has 32 protons. If you add a proton it is no longer Germanium but becomes Arsenic. ...
... The number of protons is ALWAYS the same for an atom of a specific element. Germanium ALWAYS has 32 protons. If you add a proton it is no longer Germanium but becomes Arsenic. ...
Reactions and Stoichiometry Practice Problems
... 29) Aqueous aluminum chloride and water are formed when solid aluminum oxide is submersed in hydrochloric acid. Find the mass of aluminum oxide that reacts when 0.250 moles of HCl react. ...
... 29) Aqueous aluminum chloride and water are formed when solid aluminum oxide is submersed in hydrochloric acid. Find the mass of aluminum oxide that reacts when 0.250 moles of HCl react. ...
Properties of Elements
... Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
... Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
10th Carbon and Its Compounds Solved Paper-3
... ANS [2-ii] We know that esters are sweet smelling substances which are used in perfumes. These are formed when a carboxylic acid reacts with an alcohol in presence of conc. H2SO4. Since new compound A (i.e., Ethanoic acid) reacts with ethanol (an alcohol) in presence of conc. H2SO4 to form a sweet s ...
... ANS [2-ii] We know that esters are sweet smelling substances which are used in perfumes. These are formed when a carboxylic acid reacts with an alcohol in presence of conc. H2SO4. Since new compound A (i.e., Ethanoic acid) reacts with ethanol (an alcohol) in presence of conc. H2SO4 to form a sweet s ...
New Title
... 4. Circle the letter of each sentence that is true about valence electrons and chemical bonding. a. Most atoms are less stable when they have eight valence electrons. b. Atoms with eight valence electrons easily form compounds. c. Having eight valence electrons makes atoms very reactive. d. Atoms wi ...
... 4. Circle the letter of each sentence that is true about valence electrons and chemical bonding. a. Most atoms are less stable when they have eight valence electrons. b. Atoms with eight valence electrons easily form compounds. c. Having eight valence electrons makes atoms very reactive. d. Atoms wi ...
Chapter 1
... a) rice pudding Heterogeneous mixture b) seawater Homogeneous mixture unless there are undissolved particles such as sand, then heterogeneous c) magnesium Element d) gasoline Homogeneous mixture ...
... a) rice pudding Heterogeneous mixture b) seawater Homogeneous mixture unless there are undissolved particles such as sand, then heterogeneous c) magnesium Element d) gasoline Homogeneous mixture ...
Questions on Chapter 7
... 3) Electrons in the 1s subshell are much closer to the nucleus in Ar than in He due to the larger __________ in Ar. A) nuclear charge B) paramagnetism C) diamagnetism D) Hund's rule E) azimuthal (magnetic moment) quantum number 4) Screening of the nuclear charge by core electrons in atoms is _______ ...
... 3) Electrons in the 1s subshell are much closer to the nucleus in Ar than in He due to the larger __________ in Ar. A) nuclear charge B) paramagnetism C) diamagnetism D) Hund's rule E) azimuthal (magnetic moment) quantum number 4) Screening of the nuclear charge by core electrons in atoms is _______ ...
Name: (1 of 2) Math Set # 13 Protons, Neutrons, Electrons Proton
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
Science 2nd prep. 1st term Lesson 2 Graduation of the properties of
... The ability of the oxygen atom to attract e-covalent bonds (OH) is greater than the capacity of the hydrogen atom because the electronegativity of oxygen (3.5) is greater than Alsalbeh hydrogen (2.1) Water from the polar covalent compounds, because difference electronegativity ...
... The ability of the oxygen atom to attract e-covalent bonds (OH) is greater than the capacity of the hydrogen atom because the electronegativity of oxygen (3.5) is greater than Alsalbeh hydrogen (2.1) Water from the polar covalent compounds, because difference electronegativity ...
g - Porterville College Home
... formulas. Binary molecular compounds are named using the greek prefixes. Do not allow other instances of Greek or similar prefixes to confuse use in naming some of the oxyanions. For example, Cr2O72- is named dichromate. This has nothing to do with the naming of binary molecular compounds. There are ...
... formulas. Binary molecular compounds are named using the greek prefixes. Do not allow other instances of Greek or similar prefixes to confuse use in naming some of the oxyanions. For example, Cr2O72- is named dichromate. This has nothing to do with the naming of binary molecular compounds. There are ...
Chemical Reactions
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
Colored Period Table
... The top number is the atomic number. Every element has its own unique atomic number. The atomic number tells how many protons are in one atom of that element. Since no two elements have the same atomic number, no two elements have the same number of protons. The large letter is the element's symbol, ...
... The top number is the atomic number. Every element has its own unique atomic number. The atomic number tells how many protons are in one atom of that element. Since no two elements have the same atomic number, no two elements have the same number of protons. The large letter is the element's symbol, ...
Periodic Table Vocabulary Alkali metals
... 8. Transition elements- Any of the metallic elements within Groups 3 to 12 in the Periodic Table that have an incomplete inner electron shell and that serve as transitional links between the most and the least electropositive in a series of elements. Sentence-The number of valence electrons in the t ...
... 8. Transition elements- Any of the metallic elements within Groups 3 to 12 in the Periodic Table that have an incomplete inner electron shell and that serve as transitional links between the most and the least electropositive in a series of elements. Sentence-The number of valence electrons in the t ...