formula writing and nomenclature of inorganic compounds
... numbers match and the formula of potassium bromide is correct as written above. The formula of potassium bromide is interpreted to mean that a molecule of the compound contains one atom of potassium and one atom of bromine. 2. Write the formula of iron(II) bromide. In this example, it is found that ...
... numbers match and the formula of potassium bromide is correct as written above. The formula of potassium bromide is interpreted to mean that a molecule of the compound contains one atom of potassium and one atom of bromine. 2. Write the formula of iron(II) bromide. In this example, it is found that ...
2. CHEMICAL ACTIVITY of the METALS 3. PATTERNS of the
... • Steel (in its various forms) is very hard and strong. • It can be cast, milled, rolled, worked, bent, cut and ...
... • Steel (in its various forms) is very hard and strong. • It can be cast, milled, rolled, worked, bent, cut and ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... increasing atomic number, there is a periodic repetition of their physical and chemical properties Elements that are in the same group have similar chemical and physical properties ...
... increasing atomic number, there is a periodic repetition of their physical and chemical properties Elements that are in the same group have similar chemical and physical properties ...
Chapter 5.1 History of PT - Effingham County Schools
... Dmitri Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular ...
... Dmitri Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular ...
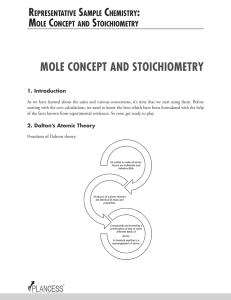
mole concept and stoichiometry
... The Law States that , “The ratio of the weights of two elements, A and B which combine separately with a fixed weight of the third element C is either the same or some simple multiple of the ratio of the weights in which A and B combine directly with each other.” He introduced the term “Stoichiometr ...
... The Law States that , “The ratio of the weights of two elements, A and B which combine separately with a fixed weight of the third element C is either the same or some simple multiple of the ratio of the weights in which A and B combine directly with each other.” He introduced the term “Stoichiometr ...
stoichiometry
... b. How much hydrogen would you need in this case? c. If you had 30 moles of H2 and 20 moles of O2, how much water could you make? d. Which substance is the limiting reagent? e. If you had 0.32moles of H2, how much water could it make and how much O2 ...
... b. How much hydrogen would you need in this case? c. If you had 30 moles of H2 and 20 moles of O2, how much water could you make? d. Which substance is the limiting reagent? e. If you had 0.32moles of H2, how much water could it make and how much O2 ...
View PDF
... ____ 17. In the reaction A + B → C + D, if the quantity of B is insufficient to react with all of A, a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a che ...
... ____ 17. In the reaction A + B → C + D, if the quantity of B is insufficient to react with all of A, a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a che ...
Chemical Equations and Reactions
... TABLE 8-1 Elements That Normally Exist as Diatomic Molecules Element ...
... TABLE 8-1 Elements That Normally Exist as Diatomic Molecules Element ...
Ch. 11-12 Supplements
... 11) Use the following equation to answer the questions below. 2 H2 + O2 2 H2O + 126 kcals a. How many moles of oxygen are needed to produce 55.0 grams of water? b. How many kilocalories of energy are produced from the reaction of 44.8 liters of hydrogen? c. If 750. kcal of energy are released, how ...
... 11) Use the following equation to answer the questions below. 2 H2 + O2 2 H2O + 126 kcals a. How many moles of oxygen are needed to produce 55.0 grams of water? b. How many kilocalories of energy are produced from the reaction of 44.8 liters of hydrogen? c. If 750. kcal of energy are released, how ...
12 - einstein classes
... is broken into small lumps and put into the ammonia convertor, where the Fe3O4 is reduced to give small crystals of iron in a refractory matrix. This is the active catalyst. The actual plant is more complicated than this one-stage reaction implies, since the N2 and H2 must be made before they can be ...
... is broken into small lumps and put into the ammonia convertor, where the Fe3O4 is reduced to give small crystals of iron in a refractory matrix. This is the active catalyst. The actual plant is more complicated than this one-stage reaction implies, since the N2 and H2 must be made before they can be ...
Year 11 C2 Mock Exam Revision Questions
... Oxygen atoms have 8 electrons. Complete the diagram to represent the arrangement of electrons in an oxygen atom. Use crosses (×) to represent the electrons. ...
... Oxygen atoms have 8 electrons. Complete the diagram to represent the arrangement of electrons in an oxygen atom. Use crosses (×) to represent the electrons. ...
IT`S ATOMIC
... considered to be nonmetals. Metals and nonmetals have very different properties. As a result, metals and nonmetals will combine to form Group Figure 1 new substances. In addition to the zigzag line, the periodic table contains vertical columns of elements as well as horizontal rows of elements. The ...
... considered to be nonmetals. Metals and nonmetals have very different properties. As a result, metals and nonmetals will combine to form Group Figure 1 new substances. In addition to the zigzag line, the periodic table contains vertical columns of elements as well as horizontal rows of elements. The ...
1)A neutral atom has no overall charge, and ion is a
... 5)a)Create graph, will be gone over in class. b)These are the smallest atoms on each of their respective rows, and electrons are being removed from filled orbitals, which have strong stability, which takes a lot of energy to do. c)The valence electrons experience a smaller nuclear force of attractio ...
... 5)a)Create graph, will be gone over in class. b)These are the smallest atoms on each of their respective rows, and electrons are being removed from filled orbitals, which have strong stability, which takes a lot of energy to do. c)The valence electrons experience a smaller nuclear force of attractio ...
chap-4-atomic-weights
... formulas for gases A, B and C. These formulas could then be used to derive atomic weights. For example, his formulas transformed Dalton’s law of multiple proportions data from “28g nitrogen + 16g oxygen 44g A” into “28g N2 + 16g O2 -> 44g N2O.” Since the product is N2O, the 28g of N2 must have twi ...
... formulas for gases A, B and C. These formulas could then be used to derive atomic weights. For example, his formulas transformed Dalton’s law of multiple proportions data from “28g nitrogen + 16g oxygen 44g A” into “28g N2 + 16g O2 -> 44g N2O.” Since the product is N2O, the 28g of N2 must have twi ...
History of the Periodic Table
... The familiar periodic table that adorns many science classrooms is based on a number of early efforts to identify and classify the elements. In the 1790’s, one of the first lists of elements and their compounds was compiled by French chemist Antioine-Laurent Lavioser. It was Lavoisier who divided th ...
... The familiar periodic table that adorns many science classrooms is based on a number of early efforts to identify and classify the elements. In the 1790’s, one of the first lists of elements and their compounds was compiled by French chemist Antioine-Laurent Lavioser. It was Lavoisier who divided th ...
AP Chemistry Chapter 7 Lecture Notes 7.1 Development
... ∆Hfo = +284.6 kJ •Ozone is pungent and toxic. •Oxygen (or dioxygen, O2) is a potent oxidizing agent since the O2– ion has a noble gas configuration. •There are two oxidation states for oxygen: –2 (e.g., H2O) and –1 (e.g., H2O2). •Sulfur is another important member of this group. •The most common for ...
... ∆Hfo = +284.6 kJ •Ozone is pungent and toxic. •Oxygen (or dioxygen, O2) is a potent oxidizing agent since the O2– ion has a noble gas configuration. •There are two oxidation states for oxygen: –2 (e.g., H2O) and –1 (e.g., H2O2). •Sulfur is another important member of this group. •The most common for ...
Name:
... elements. Scientists during Dmitri’s time were trying to figure out an easy way in which they could organize the elements of matter so that it would be easy for them to communicate about their properties (i.e. scientists, even back then, were lazy and they didn’t want to have to memorize all the ele ...
... elements. Scientists during Dmitri’s time were trying to figure out an easy way in which they could organize the elements of matter so that it would be easy for them to communicate about their properties (i.e. scientists, even back then, were lazy and they didn’t want to have to memorize all the ele ...
m03_che_sb_ibdip_9755_u03
... Periods and groups If you have visited a large supermarket you will appreciate the importance of a classification system. Similar products are grouped together to help you find what you want. In the same way, a chemist knows what type of element to find in different parts of the Periodic Table. The ...
... Periods and groups If you have visited a large supermarket you will appreciate the importance of a classification system. Similar products are grouped together to help you find what you want. In the same way, a chemist knows what type of element to find in different parts of the Periodic Table. The ...
Periodic Table of Elements
... The periodic table is a tabular display of the chemical elements. The elements are organized based on their atomic numbers, electron configurations, and recurring chemical properties. In the periodic table, elements are presented in order of increasing atomic number (the number of protons). The row ...
... The periodic table is a tabular display of the chemical elements. The elements are organized based on their atomic numbers, electron configurations, and recurring chemical properties. In the periodic table, elements are presented in order of increasing atomic number (the number of protons). The row ...
periodic classification - cpprashanths Chemistry
... 5.What are the achievements (merits) of Mendeleev’s periodic table? 1.Systematic study of elements.:- He was the first to arrange elements in to groups and periods which made the study of elements simple. 2.Correction of doubtful atomic masses.:-His periodic table helped in correcting the doubtful ...
... 5.What are the achievements (merits) of Mendeleev’s periodic table? 1.Systematic study of elements.:- He was the first to arrange elements in to groups and periods which made the study of elements simple. 2.Correction of doubtful atomic masses.:-His periodic table helped in correcting the doubtful ...
Topic 7b Redox notes
... CuO is losing oxygen and so is reduced. This happens when heated with hydrogen. Hydrogen has reduced CuO to copper metal and has itself gained oxygen and therefore been oxidised. You could also consider the oxidation states of each substance and would draw the same conclusion. ...
... CuO is losing oxygen and so is reduced. This happens when heated with hydrogen. Hydrogen has reduced CuO to copper metal and has itself gained oxygen and therefore been oxidised. You could also consider the oxidation states of each substance and would draw the same conclusion. ...
File
... useful exercises for people to perform and use when trying to give body to their own imagination.” What do you think Levi means in this quotation? Is there some way you could use the properties of an element as a metaphor? ...
... useful exercises for people to perform and use when trying to give body to their own imagination.” What do you think Levi means in this quotation? Is there some way you could use the properties of an element as a metaphor? ...
Topic 5 - Holy Cross Collegiate
... useful exercises for people to perform and use when trying to give body to their own imagination.” What do you think Levi means in this quotation? Is there some way you could use the properties of an element as a metaphor? ...
... useful exercises for people to perform and use when trying to give body to their own imagination.” What do you think Levi means in this quotation? Is there some way you could use the properties of an element as a metaphor? ...
Document
... Make up 2/3 of the periodic table Low ionization energy: amount of energy needed to remove valence electron Low electronegativity: desire for more electrons. Solid at room temperature except mercury (Hg) Good conductors of heat and electricity Malleable – can be hammered into shapes (thin ...
... Make up 2/3 of the periodic table Low ionization energy: amount of energy needed to remove valence electron Low electronegativity: desire for more electrons. Solid at room temperature except mercury (Hg) Good conductors of heat and electricity Malleable – can be hammered into shapes (thin ...