Periodic Table Student Outline
... Fond of card games, he wrote the weight for each element on a separate index card and sorted them as in solitaire. Elements with similar properties formed a “suit” that he placed in columns ordered by ascending atomic weight. Now he had a new Periodic Law (“Elements arranged according to the value o ...
... Fond of card games, he wrote the weight for each element on a separate index card and sorted them as in solitaire. Elements with similar properties formed a “suit” that he placed in columns ordered by ascending atomic weight. Now he had a new Periodic Law (“Elements arranged according to the value o ...
Chemistry JAMB Past Questions
... decreases the density of the gas decreases the temperature of the gas increases the density of the gas increases the volume of the gas. 2.5 g of a hydrated barium salt gave on heating, 2.13 g of the anhydrous salt. Given that the relative molecular mass of the anhydrous salt is 208, the number of mo ...
... decreases the density of the gas decreases the temperature of the gas increases the density of the gas increases the volume of the gas. 2.5 g of a hydrated barium salt gave on heating, 2.13 g of the anhydrous salt. Given that the relative molecular mass of the anhydrous salt is 208, the number of mo ...
C:\SUBJECTS\SUBJECTS\Chemistry
... E. Ion (T), copper (L), aluminium (TL). In the preparation of some pure crystals of Cu (NO3)2 starting with CuO, a student gave the following statements as steps he employed. Which of these shows a flaw in his report? A. Some CuO was reacted with excess dilute H2SO4 B. The solution was concentrated ...
... E. Ion (T), copper (L), aluminium (TL). In the preparation of some pure crystals of Cu (NO3)2 starting with CuO, a student gave the following statements as steps he employed. Which of these shows a flaw in his report? A. Some CuO was reacted with excess dilute H2SO4 B. The solution was concentrated ...
Chem.-Chapter-6-notes
... What are the trends among the elements for atomic size? In general, atomic size increases from top to bottom within a group and decreases from left to right across a period. How do ions form? Positive and negative ions form when electrons are transferred between atoms. What are the trends among the ...
... What are the trends among the elements for atomic size? In general, atomic size increases from top to bottom within a group and decreases from left to right across a period. How do ions form? Positive and negative ions form when electrons are transferred between atoms. What are the trends among the ...
H Unit 4: Periodic Table
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
periodic table - Cloudfront.net
... • The electron cloud is 100,000 times larger than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact locati ...
... • The electron cloud is 100,000 times larger than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact locati ...
Ch. 17 PPT
... • The electron cloud is 100,000 times larger than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact locati ...
... • The electron cloud is 100,000 times larger than the diameter of the nucleus. • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact locati ...
9/98 scerri 7p dom - PubContent test page
... by what scientists call quantum numbers. The lengths of the various periods emerge from experimental evidence about the order of electron-shell filling and from the quantum-mechanical restrictions on the four quantum numbers that electrons can adopt. The modifications to quantum theory made by Werne ...
... by what scientists call quantum numbers. The lengths of the various periods emerge from experimental evidence about the order of electron-shell filling and from the quantum-mechanical restrictions on the four quantum numbers that electrons can adopt. The modifications to quantum theory made by Werne ...
Addrienne`s Element Lesson Plan
... including all of the requested information; showed a clear understanding of atomic number, valence electrons, and electron shells by placing their cards in the correct order; worked well within their group to identify several common properties of elements within their assigned family. 2 points: Stud ...
... including all of the requested information; showed a clear understanding of atomic number, valence electrons, and electron shells by placing their cards in the correct order; worked well within their group to identify several common properties of elements within their assigned family. 2 points: Stud ...
Chemical Elements Essay Research Paper At first
... weight of the tellurium and iodine had made an error in calculating it. For that reason his placement of the two elements was incorrect. But this time, Mendeleev was wrong. The correct explanation for this apparent confusion did not appear for nearly 50 years. Then the English physicist H.G. J. Mose ...
... weight of the tellurium and iodine had made an error in calculating it. For that reason his placement of the two elements was incorrect. But this time, Mendeleev was wrong. The correct explanation for this apparent confusion did not appear for nearly 50 years. Then the English physicist H.G. J. Mose ...
Document
... elements. Scientists during Dmitri’s time were trying to figure out an easy way in which they could organize the elements of matter so that it would be easy for them to communicate about their properties (i.e. scientists, even back then, were lazy and they didn’t want to have to memorize all the ele ...
... elements. Scientists during Dmitri’s time were trying to figure out an easy way in which they could organize the elements of matter so that it would be easy for them to communicate about their properties (i.e. scientists, even back then, were lazy and they didn’t want to have to memorize all the ele ...
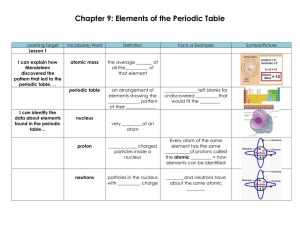
Chapter 9: Elements of the Periodic Table
... never found as uncombined elements in __________ shiny and so _______can cut with a plastic knife have low __________ and ___________ points ...
... never found as uncombined elements in __________ shiny and so _______can cut with a plastic knife have low __________ and ___________ points ...
Introduction The Periodic Law
... Metalloids have properties of both metals and nonmetals. Metalloids can be shiny or dull. Electricity and heat can travel through metalloids, although not as easily as they can through metals. They are also called semi-metals. They are typically semiconductors, which means that they conduct electric ...
... Metalloids have properties of both metals and nonmetals. Metalloids can be shiny or dull. Electricity and heat can travel through metalloids, although not as easily as they can through metals. They are also called semi-metals. They are typically semiconductors, which means that they conduct electric ...
Periodic Table Packet
... 1. Color a box around each of the alkali metals, atomic numbers 3, 11, 19, 37,55, and 87 red, because they are wildly reactive. Color lightest at the top through darkest at the bottom to indicate the increasing reactivity of the group members. 2. Color a box around each of the alkaline earth metals, ...
... 1. Color a box around each of the alkali metals, atomic numbers 3, 11, 19, 37,55, and 87 red, because they are wildly reactive. Color lightest at the top through darkest at the bottom to indicate the increasing reactivity of the group members. 2. Color a box around each of the alkaline earth metals, ...
Solution - Welcome To Badhan Education
... The ionization enthalpies of the two isotopes of an element are expected to be same because the isotopes have same electronic configuration and same nuclear charge. 24. What are the major differences between metals and non-metals? ...
... The ionization enthalpies of the two isotopes of an element are expected to be same because the isotopes have same electronic configuration and same nuclear charge. 24. What are the major differences between metals and non-metals? ...
Thermodynamics Practice Problems Presentation
... 1 mol each of H–H and F–F bonds are broken The bonds formed are 2 mol of H–F bonds ∆H= (nH-HDH-H + nF-FDF-F) – nH-FDH-F (1 mol x 432KJ) + (1 mol x 154 KJ) - (2 mol x 565 KJ mol mol mol ∆H = -544 KJ The enthalpy change for the reaction of 1 mol hydrogen gas and 1 mol fluorine gas to ptoduce 2 mol. Hy ...
... 1 mol each of H–H and F–F bonds are broken The bonds formed are 2 mol of H–F bonds ∆H= (nH-HDH-H + nF-FDF-F) – nH-FDH-F (1 mol x 432KJ) + (1 mol x 154 KJ) - (2 mol x 565 KJ mol mol mol ∆H = -544 KJ The enthalpy change for the reaction of 1 mol hydrogen gas and 1 mol fluorine gas to ptoduce 2 mol. Hy ...
Electrons in Atoms - Effingham County Schools
... aluminum, gallium, indium, tin, thallium, lead, bismuth ...
... aluminum, gallium, indium, tin, thallium, lead, bismuth ...
Isotopes and the Electron Configuration of the Blocks in the Periodic
... It is known that elements of the Periodic Table of Elements have fractional numerical values of atomic masses. This is because the elements consists of, as regularly, a mix of inborn (native) isotopes. For this reason we conclude that the average weighted atomic mass of all stable isotopes of any el ...
... It is known that elements of the Periodic Table of Elements have fractional numerical values of atomic masses. This is because the elements consists of, as regularly, a mix of inborn (native) isotopes. For this reason we conclude that the average weighted atomic mass of all stable isotopes of any el ...
Chapter 5 The Periodic Table Section 1 Organizing the Elements
... > What are the three main categories of elements? > All elements are either metals, nonmetals, or semiconductors. • Elements in each category have similar properties. – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element conducts heat and electricity po ...
... > What are the three main categories of elements? > All elements are either metals, nonmetals, or semiconductors. • Elements in each category have similar properties. – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element conducts heat and electricity po ...
Chapter 7 The Development of the Periodic Table
... share electrons when forming compounds. • Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
... share electrons when forming compounds. • Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. ...
Types of Chemical Reactions
... 1. Masses give information about # of p+, n0, e– 2. It is useful to know relative mass E.g. Q - What ratio is needed to make H2O? A - 2:1 by atoms, but 2:16 by mass It is useful to associate atomic mass with a mass in grams. It has been found that 1g H, 12g C, or 23g Na have 6.02x1023 atoms 6.02 ...
... 1. Masses give information about # of p+, n0, e– 2. It is useful to know relative mass E.g. Q - What ratio is needed to make H2O? A - 2:1 by atoms, but 2:16 by mass It is useful to associate atomic mass with a mass in grams. It has been found that 1g H, 12g C, or 23g Na have 6.02x1023 atoms 6.02 ...
Periods and Blocks of the Periodic Table
... • beryllium, magnesium, calcium, strontium, barium, and radium • Group 2 metals are less reactive than the alkali metals, but are still too reactive to be found in nature in pure form. ...
... • beryllium, magnesium, calcium, strontium, barium, and radium • Group 2 metals are less reactive than the alkali metals, but are still too reactive to be found in nature in pure form. ...
Science 2nd prep 1st term 1st lesson Many attempts are made by
... in the modern periodic table by knowing their atomic numbers and vice versa. *The elements of (B) groups lie in the middle of the table. *Recently discovered elements are not found in nature but they are prepared artificially. These elements are radioactive elements, their nuclei are decayed in less ...
... in the modern periodic table by knowing their atomic numbers and vice versa. *The elements of (B) groups lie in the middle of the table. *Recently discovered elements are not found in nature but they are prepared artificially. These elements are radioactive elements, their nuclei are decayed in less ...
高雄醫學大學九十二學年度學士後醫學系招生考試試題 科目:化學 考試
... (B) No precipitate forms. (C) CuS will precipitate from solution (D) NaCl will precipitate from solution. (E) No reaction will occur. 65. Body temperature is about 308 K. On a cold day, what volume of air at 273 K must a person with a lung capacity of 2.00 L breathe in to fill up the lungs? (A) 1.13 ...
... (B) No precipitate forms. (C) CuS will precipitate from solution (D) NaCl will precipitate from solution. (E) No reaction will occur. 65. Body temperature is about 308 K. On a cold day, what volume of air at 273 K must a person with a lung capacity of 2.00 L breathe in to fill up the lungs? (A) 1.13 ...