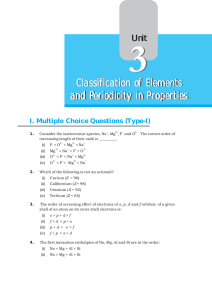
Valence Electrons - Warren County Public Schools
... Periodic Table: 4.30.14 Objectives: •Periodic Table Exam •I can predict chemical reactivity for an element based on its number of valence electrons and location on periodic table. • I can distinguish between metallic and non-metallic properties. •I can graph and interpret periodic trends. •I can un ...
... Periodic Table: 4.30.14 Objectives: •Periodic Table Exam •I can predict chemical reactivity for an element based on its number of valence electrons and location on periodic table. • I can distinguish between metallic and non-metallic properties. •I can graph and interpret periodic trends. •I can un ...
formula writing and nomenclature of inorganic - Parkway C-2
... share electrons in forming a chemical bond. The number of electrons that an atom loses, gains, or shares when it bonds with another atom is known as the oxidation number of the atom. Elements which lose electrons in a chemical reaction, or which have electrons which are shared with another element d ...
... share electrons in forming a chemical bond. The number of electrons that an atom loses, gains, or shares when it bonds with another atom is known as the oxidation number of the atom. Elements which lose electrons in a chemical reaction, or which have electrons which are shared with another element d ...
11-chemistry-exemplar-chapter-3
... Thus process of formation of O in gas phase is unfavourable even though O 2– is isoelectronic with neon. It is due to the fact that, (i) ...
... Thus process of formation of O in gas phase is unfavourable even though O 2– is isoelectronic with neon. It is due to the fact that, (i) ...
Unit 1 Answer Key
... 8. When elements are arranged by atomic number, their chemical and physical properties recur periodically. Many elements have similar properties and these properties follow a pattern that repeats itself regularly. 9. Each column in the periodic table constitutes a group. Groups contain elements ...
... 8. When elements are arranged by atomic number, their chemical and physical properties recur periodically. Many elements have similar properties and these properties follow a pattern that repeats itself regularly. 9. Each column in the periodic table constitutes a group. Groups contain elements ...
d) Ramsay. The idea of arranging the elements in the periodic table
... The ionization energies for removing successive electrons from sodium are 496 kJ/mol, 4562 kJ/mol, 6912 kJ/mol, and 9544 kJ/mol. The great jump in ionization energy after the first electron is removed indicates that a) sodium has four or five electrons. ...
... The ionization energies for removing successive electrons from sodium are 496 kJ/mol, 4562 kJ/mol, 6912 kJ/mol, and 9544 kJ/mol. The great jump in ionization energy after the first electron is removed indicates that a) sodium has four or five electrons. ...
Element Project - Dover Bay
... really hyper and active. He’s in the Gas Team, along with some of his other friends, like Helium and Neon. They’re always exercising and their atoms are moving around freely and independently. Hydrogen is also a really cool and friendly guy because he’s extremely reactive. He has only one valence el ...
... really hyper and active. He’s in the Gas Team, along with some of his other friends, like Helium and Neon. They’re always exercising and their atoms are moving around freely and independently. Hydrogen is also a really cool and friendly guy because he’s extremely reactive. He has only one valence el ...
answer ch6 - Mr Khaled Nasr
... The number of atoms, molecules or ions in one mole of any substance. A unit of quantity that consists of 6.02 x 1023 particles. The mass in grams of one mole of any pure substance. The volumes of gases involved in a reaction and the gases produced exist in fixed ratios. The law which states that equ ...
... The number of atoms, molecules or ions in one mole of any substance. A unit of quantity that consists of 6.02 x 1023 particles. The mass in grams of one mole of any pure substance. The volumes of gases involved in a reaction and the gases produced exist in fixed ratios. The law which states that equ ...
Stoichiometry1
... reacts with two moles of oxygen to produce two moles of water and one mole of carbon dioxide gas. ...
... reacts with two moles of oxygen to produce two moles of water and one mole of carbon dioxide gas. ...
Differentiated Chemistry Worksheet and Laboratory
... We spend quite a bit of time staring at the red and green lights in a traffic signals. While that image is in your mind, for questions 92 – 95, decide which would have the following for a red light with a 4.41 x 1014 Hz frequency or a green light with a 6.00 x 1014 Hz frequency. Explain. ...
... We spend quite a bit of time staring at the red and green lights in a traffic signals. While that image is in your mind, for questions 92 – 95, decide which would have the following for a red light with a 4.41 x 1014 Hz frequency or a green light with a 6.00 x 1014 Hz frequency. Explain. ...
This article was published in an Elsevier journal. The attached copy
... an easy phase separation. In the subsequent step, Section 3 including reactions (9) and (10), the separation of HI from L − 2, the heavier iodine/iodide–water phase, is the most critical scenario of the cycle [4] and believed to be the most expensive and energy-consuming step [5]. After establishing ...
... an easy phase separation. In the subsequent step, Section 3 including reactions (9) and (10), the separation of HI from L − 2, the heavier iodine/iodide–water phase, is the most critical scenario of the cycle [4] and believed to be the most expensive and energy-consuming step [5]. After establishing ...
Atomic
... from the draft version to reflect the changes made in the accredited specification. These changes are also reflected in the learning outcomes with some additions to the resources. The scheme of work is designed to be a flexible medium term plan for teaching content and development of the skills that ...
... from the draft version to reflect the changes made in the accredited specification. These changes are also reflected in the learning outcomes with some additions to the resources. The scheme of work is designed to be a flexible medium term plan for teaching content and development of the skills that ...
The Periodic Table
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
Class-XII, Summer assignment
... Ans; H2O>H2S>H2Se>H2Te>H2Po As atomic size increases E—H bond strength decreases, Hence thermal stability decreases. 26. Why is H2O a liquid and H2S a gas ? Ans: Because of small size and high electro negativity of oxygen, molecules of water are highly associated through hydrogen bonding resulting i ...
... Ans; H2O>H2S>H2Se>H2Te>H2Po As atomic size increases E—H bond strength decreases, Hence thermal stability decreases. 26. Why is H2O a liquid and H2S a gas ? Ans: Because of small size and high electro negativity of oxygen, molecules of water are highly associated through hydrogen bonding resulting i ...
5.2 The Modern Periodic Table
... To understand structure of table, think about what happens as atomic number increases. n atomic number = # of protons n How does this relate to # of electrons in an ...
... To understand structure of table, think about what happens as atomic number increases. n atomic number = # of protons n How does this relate to # of electrons in an ...
Answers - Pearson-Global
... example) of water with the coloured liquids introduced into the bottom of them. A simple observation of the progress of the colours up the tubes would be enough. There could be some problems if the liquids varied markedly in colour intensity. A student suggesting that you might put some white card o ...
... example) of water with the coloured liquids introduced into the bottom of them. A simple observation of the progress of the colours up the tubes would be enough. There could be some problems if the liquids varied markedly in colour intensity. A student suggesting that you might put some white card o ...
Unit Six: Atomic structure
... 1. Dimitri Mendeleev , a Russian scientist, is credits for creating the first periodic table in 1869. At that time, there were only 60 known element and Mendeleev organized them according to their atomic mass. Mendeleev was able to predict the properties of missing elements which were later discover ...
... 1. Dimitri Mendeleev , a Russian scientist, is credits for creating the first periodic table in 1869. At that time, there were only 60 known element and Mendeleev organized them according to their atomic mass. Mendeleev was able to predict the properties of missing elements which were later discover ...
Mendeleef`s Periodic Table
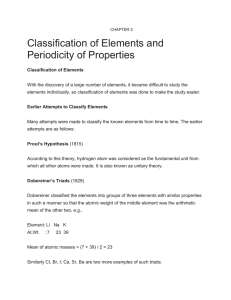
... Many attempts were made to classify the known elements from time to time. The earlier attempts are as follows: Prout’s Hypothesis (1815) According to this theory, hydrogen atom was considered as the fundamental unit from which all other atoms were made. It is also known as unitary theory. Dobereiner ...
... Many attempts were made to classify the known elements from time to time. The earlier attempts are as follows: Prout’s Hypothesis (1815) According to this theory, hydrogen atom was considered as the fundamental unit from which all other atoms were made. It is also known as unitary theory. Dobereiner ...
Chem Ch 5 Release Test
... ____ 97. As you move down the periodic table from carbon through lead, atomic radii a. generally increase. c. do not change. b. generally decrease. d. vary unpredictably. ____ 98. As you move left to right from gallium through bromine, atomic radii a. generally increase. c. do not change. b. genera ...
... ____ 97. As you move down the periodic table from carbon through lead, atomic radii a. generally increase. c. do not change. b. generally decrease. d. vary unpredictably. ____ 98. As you move left to right from gallium through bromine, atomic radii a. generally increase. c. do not change. b. genera ...
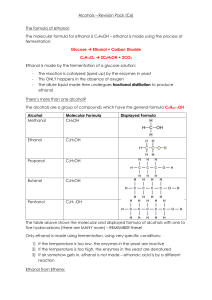
C6 Revision Guide - West Derby School
... Alcohols – Revision Pack (C6) Washing machines have to heat up a lot of water – this requires ENERGY, so the lower the temperature of the water, the less energy is used and smaller volumes of greenhouse gases are put into the atmosphere. Washing clothes at low temperatures is also good for coloured ...
... Alcohols – Revision Pack (C6) Washing machines have to heat up a lot of water – this requires ENERGY, so the lower the temperature of the water, the less energy is used and smaller volumes of greenhouse gases are put into the atmosphere. Washing clothes at low temperatures is also good for coloured ...
Chapter 1 questions
... An oxide of sulfur contains 40.0% by mass of sulfur. Calculate the empirical formula of the oxide. Q8. Analysis by mass has indicated the following percentage composition by mass of certain compounds. Calculate the empirical formula of each: a) carbon 75.0%, hydrogen 25.0% b) magnesium 60.3%, oxygen ...
... An oxide of sulfur contains 40.0% by mass of sulfur. Calculate the empirical formula of the oxide. Q8. Analysis by mass has indicated the following percentage composition by mass of certain compounds. Calculate the empirical formula of each: a) carbon 75.0%, hydrogen 25.0% b) magnesium 60.3%, oxygen ...