The Periodic Table
... • Since the properties of the elements follow regular patterns, we can predict unknown properties of elements based on those around it. • For example, table 6.2 lists several properties of the alkali metals except francium, Fr. • We can predict the properties of francium based on the other alkali me ...
... • Since the properties of the elements follow regular patterns, we can predict unknown properties of elements based on those around it. • For example, table 6.2 lists several properties of the alkali metals except francium, Fr. • We can predict the properties of francium based on the other alkali me ...
First Term Science Al-Karma Language School Prep 2 Question (1
... weight, while Moseley arranged them ascending according to atomic number. 10)-Calcium (Ca) and Magnesium (Mg) elements are examples of alkaline earth metals. 11)-The valency energy level of halogen contains seven electrons, while that of alkaline earth metal has two electrons. 12)-Sodium and potassi ...
... weight, while Moseley arranged them ascending according to atomic number. 10)-Calcium (Ca) and Magnesium (Mg) elements are examples of alkaline earth metals. 11)-The valency energy level of halogen contains seven electrons, while that of alkaline earth metal has two electrons. 12)-Sodium and potassi ...
Earth Science Chapter 4: Minerals Chapter Overview Section 1
... • Hardness – One of the most useful tests for identifying minerals is hardness. Hardness is a measure of how easily a mineral can be scratched. Mohs scale of hardness uses the hardness of ten known minerals to compare with the hardness of unknown minerals. Talc, the softest mineral, has a hardness o ...
... • Hardness – One of the most useful tests for identifying minerals is hardness. Hardness is a measure of how easily a mineral can be scratched. Mohs scale of hardness uses the hardness of ten known minerals to compare with the hardness of unknown minerals. Talc, the softest mineral, has a hardness o ...
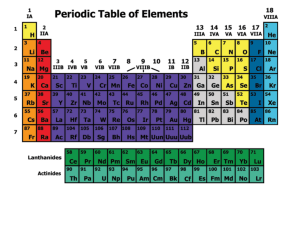
Station 1: The Periodic Table 1a. Students know how to relate the
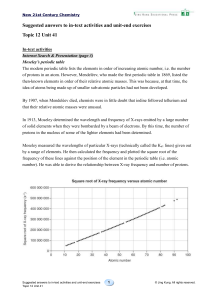
... Ionization energy increases from left to right and decreases from top to bottom. Inonization energy (IE) is the energy required to remove an electron. o IE increases from left to right because the elements increase in nuclear charge in this direction. o IE decreases from top to bottom because as ...
... Ionization energy increases from left to right and decreases from top to bottom. Inonization energy (IE) is the energy required to remove an electron. o IE increases from left to right because the elements increase in nuclear charge in this direction. o IE decreases from top to bottom because as ...
The Periodic Table
... at regular intervals —like the appearance of Haley’s comet every 76 years ...
... at regular intervals —like the appearance of Haley’s comet every 76 years ...
The Periodic Table: Chapter Problems Periodic Table Class Work
... 13. To which group on the periodic table does magnesium belong? 14. To which group on the periodic table does bromine belong? 15. A mystery metal is in the same period as sulfur. It has a larger atomic number than sulfur and is nonreactive. What is the mystery element? 16. Two elements are studied. ...
... 13. To which group on the periodic table does magnesium belong? 14. To which group on the periodic table does bromine belong? 15. A mystery metal is in the same period as sulfur. It has a larger atomic number than sulfur and is nonreactive. What is the mystery element? 16. Two elements are studied. ...
Periodic Table Element Pattern
... Elemental ideas from ancient times People have known about some chemical elements such as gold, silver and copper from antiquity, as these can all be discovered in nature in native form and are relatively simple to mine with primitive tools.[2] About 330 B.C Aristotle proposed that everything is mad ...
... Elemental ideas from ancient times People have known about some chemical elements such as gold, silver and copper from antiquity, as these can all be discovered in nature in native form and are relatively simple to mine with primitive tools.[2] About 330 B.C Aristotle proposed that everything is mad ...
(halogens group) 4-write down the electronic configuration
... 4-The density of pure water in solid state is………... a-less than its density in liquid state b- equal to its density in liquid state c- equal to its density in gaseous state d- greater than its density in liquid state 5-The density of pure water in solid state is ……… liquid state a-less than b- equal ...
... 4-The density of pure water in solid state is………... a-less than its density in liquid state b- equal to its density in liquid state c- equal to its density in gaseous state d- greater than its density in liquid state 5-The density of pure water in solid state is ……… liquid state a-less than b- equal ...
Daily 40 no. – 15 John Newlands
... create an early periodic table. His organization led to the modern periodic table. He realized that every eighth element had similar characteristics. His incomplete table predicted other undiscovered elements. -Eric John Newlands was born in 1837 and died in 1898. He is known for creating the first ...
... create an early periodic table. His organization led to the modern periodic table. He realized that every eighth element had similar characteristics. His incomplete table predicted other undiscovered elements. -Eric John Newlands was born in 1837 and died in 1898. He is known for creating the first ...
The Periodic Table - Science
... Atomic Symbol: The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen," etc.). These symbols are used every where in the world Usually, a symbol is the abbreviation of the element or the abbreviated Latin name of the element. ...
... Atomic Symbol: The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen," etc.). These symbols are used every where in the world Usually, a symbol is the abbreviation of the element or the abbreviated Latin name of the element. ...
CSCOPE Periodic Table Powerpoint
... with others to form compounds Outermost level does not usually fill completely with electrons ...
... with others to form compounds Outermost level does not usually fill completely with electrons ...
The Periodic Table
... with others to form compounds Outermost level does not usually fill completely with electrons ...
... with others to form compounds Outermost level does not usually fill completely with electrons ...
Ex. 41 Answer
... The elements found by chemists before Seaborg were discovered — they existed on Earth already. The elements found by Seaborg were actually made — they are elements that do not exist naturally on Earth. Scientists found that when they fired neutrons at uranium atoms, one would occasionally stick to a ...
... The elements found by chemists before Seaborg were discovered — they existed on Earth already. The elements found by Seaborg were actually made — they are elements that do not exist naturally on Earth. Scientists found that when they fired neutrons at uranium atoms, one would occasionally stick to a ...
Periodic Classification of Elements
... If the elements are arranged sequentially in the increasing order of their atomic weights, every eighth element is having the similar properties to that of the first element like in the octave of music. This is called "Newland's Concept of Octaves". Eg: 1 ...
... If the elements are arranged sequentially in the increasing order of their atomic weights, every eighth element is having the similar properties to that of the first element like in the octave of music. This is called "Newland's Concept of Octaves". Eg: 1 ...
10TH CLASSIFICATION OF ELEMENTS CHEMISRY As a large
... 4. Metallic and nonmetallic character:- The numbert of valence electrons in an atom of the element also tells us whether the element is a metal or a nonmetal. If an atom of the element contains 1, 2 or 3 valence electrons, the element is a metal. Onm the other hand, if the atom contains 4 or more va ...
... 4. Metallic and nonmetallic character:- The numbert of valence electrons in an atom of the element also tells us whether the element is a metal or a nonmetal. If an atom of the element contains 1, 2 or 3 valence electrons, the element is a metal. Onm the other hand, if the atom contains 4 or more va ...
LessonPlans_BowmanC_Sci8th_Dec 5 Teacher Bowman Class 8th
... atomic mass, number of protons, neutrons, and electrons in an atom of an element using the periodic table. ...
... atomic mass, number of protons, neutrons, and electrons in an atom of an element using the periodic table. ...
Groups
... Group 8A or 18: Noble Gases cont. Properties 1. all are gases at room temp 2. relatively unreactive Part of the p -block They have no common ionic charge because they don’t form ions, have stable electron ...
... Group 8A or 18: Noble Gases cont. Properties 1. all are gases at room temp 2. relatively unreactive Part of the p -block They have no common ionic charge because they don’t form ions, have stable electron ...
Regents Chemistry NOTE PACKET
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
P-BLOCK ELEMENTS
... are very high. This is why these elements generally do not form trivalent (M 3 ) cations. ...
... are very high. This is why these elements generally do not form trivalent (M 3 ) cations. ...
TEST-Periodic Table
... 29. Classifying Classify the elements in Figure 5-3 as metals, metalloids, or nonmetals. Explain your answer. a. These elements are all metals. They are found on the left side of the periodic table. b. These elements are all metals. They are found on the right side of the periodic table. c. These el ...
... 29. Classifying Classify the elements in Figure 5-3 as metals, metalloids, or nonmetals. Explain your answer. a. These elements are all metals. They are found on the left side of the periodic table. b. These elements are all metals. They are found on the right side of the periodic table. c. These el ...
Appendices: Cluster 2 Atoms and Elements
... • The alchemists were the first people to perform handson experimentation. They were part philosopher, mystic, magician, and chemist. • They had three main beliefs: i. They believed that some elements could be changed into others. They attempted to change base metals (lead, tin) into valuable ones l ...
... • The alchemists were the first people to perform handson experimentation. They were part philosopher, mystic, magician, and chemist. • They had three main beliefs: i. They believed that some elements could be changed into others. They attempted to change base metals (lead, tin) into valuable ones l ...
Section 6.1 Development of the Modern Periodic Table
... • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alkaline earth metals are in group 2, and are also highly reactive. ...
... • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alkaline earth metals are in group 2, and are also highly reactive. ...
Untitled
... • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alkaline earth metals are in group 2, and are also highly reactive. ...
... • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alkaline earth metals are in group 2, and are also highly reactive. ...
Periodic Table Presentation Lesson
... Nonmetals are dull and poor conductors of heat and electric current. The solid nonmetals tend to be brittle and unmalleable. The most reactive of the nonmetals are found on the far right of the periodic table (group 17). ...
... Nonmetals are dull and poor conductors of heat and electric current. The solid nonmetals tend to be brittle and unmalleable. The most reactive of the nonmetals are found on the far right of the periodic table (group 17). ...
Name Pre-Test : Atomic Structure and the Periodic Table
... Directions: Fill in the blank with the correct word from the list at the bottom of the page. Not all words from the list will be used. 1. Atomic ________________________ refers to the arrangement and number of smaller particles in an atom. 2. The ________________________ is the center or core of an ...
... Directions: Fill in the blank with the correct word from the list at the bottom of the page. Not all words from the list will be used. 1. Atomic ________________________ refers to the arrangement and number of smaller particles in an atom. 2. The ________________________ is the center or core of an ...