Chemistr.e1a.chapter.4.new2015
... • The following reaction that you have seen before in class and the laboratory is neither a precipitation reaction nor an acid-base reaction. Cu (s) + ½ O2 (g) " CuO (s) The reaction above is one where electrons are transferred from one element to another during the reaction. This kind of reaction i ...
... • The following reaction that you have seen before in class and the laboratory is neither a precipitation reaction nor an acid-base reaction. Cu (s) + ½ O2 (g) " CuO (s) The reaction above is one where electrons are transferred from one element to another during the reaction. This kind of reaction i ...
CHAPTER 4: AQUEOUS REACTIONS AND SOLUTION
... called an electrolyte because it will allow electric current to flow through it. Example: NaCl A substance that does not form ions in solution is called a nonelectrolyte. Example: C12H22O11 ...
... called an electrolyte because it will allow electric current to flow through it. Example: NaCl A substance that does not form ions in solution is called a nonelectrolyte. Example: C12H22O11 ...
Chemical bond - Physical Science
... • Metals in Group 1A tend to lose their 1 valence electron • Metals in Group 2A tend to lose their 2 valence electrons • Metals and Metalloids in Group 3A tend to lose their 3 valence electrons • Elements in Group 4A do not tend to form ions • Nonmetals in Group 5A tend to gain 3 valence electrons • ...
... • Metals in Group 1A tend to lose their 1 valence electron • Metals in Group 2A tend to lose their 2 valence electrons • Metals and Metalloids in Group 3A tend to lose their 3 valence electrons • Elements in Group 4A do not tend to form ions • Nonmetals in Group 5A tend to gain 3 valence electrons • ...
Nugget
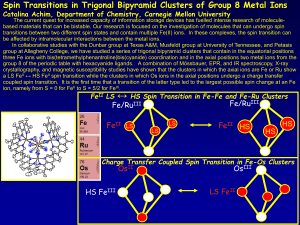
... The current quest for increased capacity of information storage devices has fuelled intense research of moleculebased materials that can be bistable. Our research is focused on the investigation of molecules that can undergo spin transitions between two different spin states and contain multiple Fe( ...
... The current quest for increased capacity of information storage devices has fuelled intense research of moleculebased materials that can be bistable. Our research is focused on the investigation of molecules that can undergo spin transitions between two different spin states and contain multiple Fe( ...
in-class assignment - hrsbstaff.ednet.ns.ca
... acetate in the solution to turn to a solid. At that point heat is released. The reason is that molecules in a liquid are moving around, but molecules in a solid are not moving around. Motion is energy, more specifically kinetic energy. When these molecules come to an abrupt halt as they go from liqu ...
... acetate in the solution to turn to a solid. At that point heat is released. The reason is that molecules in a liquid are moving around, but molecules in a solid are not moving around. Motion is energy, more specifically kinetic energy. When these molecules come to an abrupt halt as they go from liqu ...
Chapter 4: Solution Chemistry and the Hydrosphere
... To balance and complete the precipitation reactions: 1. Exchange the anions, writing the formulas for the products based on the charges of the ions! 2. Use the Solubility Rules to determine if each product is soluble or insoluble. – If at least one product is insoluble, a precipitation reaction has ...
... To balance and complete the precipitation reactions: 1. Exchange the anions, writing the formulas for the products based on the charges of the ions! 2. Use the Solubility Rules to determine if each product is soluble or insoluble. – If at least one product is insoluble, a precipitation reaction has ...
Chapter 7 Ionic and Metallic Bonding
... All atoms react to try and achieve a noble gas configuration. Noble gases have 2 s and 6 p electrons. 8 valence electrons = already stable! This is the octet rule (8 in the outer level is particularly stable). ...
... All atoms react to try and achieve a noble gas configuration. Noble gases have 2 s and 6 p electrons. 8 valence electrons = already stable! This is the octet rule (8 in the outer level is particularly stable). ...
The s-Block Elements
... 2. For Group II sulphates, the cations are much smaller than the anions. The changing in size of cations does not cause a significant change in H lattice (proportional to 1/(r+ + r-). However, the changing in size of cations does cause H hydration (proportional to 1/r+ and 1/r-) to become less exo ...
... 2. For Group II sulphates, the cations are much smaller than the anions. The changing in size of cations does not cause a significant change in H lattice (proportional to 1/(r+ + r-). However, the changing in size of cations does cause H hydration (proportional to 1/r+ and 1/r-) to become less exo ...
Bonding - Graham ISD
... is the noble gases (group 18). This is true because compounds of these atoms are almost always less stable than the original atom. Atoms with a partially stable outer energy level can lose, gain, or share electrons to obtain a stable outer energy level. ...
... is the noble gases (group 18). This is true because compounds of these atoms are almost always less stable than the original atom. Atoms with a partially stable outer energy level can lose, gain, or share electrons to obtain a stable outer energy level. ...
Chapter 4 Packet
... 2. predict whether a substance is a nonelectrolyte, strong electrolyte, or a weak electrolyte. I will also be able to predict the ions formed by electrolytes when they dissociate or ionize. 3. use Coulomb’s Law to help describe the interactions of ions during the dissolving process. 4. use solubilit ...
... 2. predict whether a substance is a nonelectrolyte, strong electrolyte, or a weak electrolyte. I will also be able to predict the ions formed by electrolytes when they dissociate or ionize. 3. use Coulomb’s Law to help describe the interactions of ions during the dissolving process. 4. use solubilit ...
Chemistry II Aqueous Reactions and Solution Chemistry Chapter 4
... the anions are surrounded by the water molecules so that the hydrogen side of the molecule surrounds the anion. The cations are surrounded by the oxygen side of the water molecule. This configuration stabilizes the ions in solution. ...
... the anions are surrounded by the water molecules so that the hydrogen side of the molecule surrounds the anion. The cations are surrounded by the oxygen side of the water molecule. This configuration stabilizes the ions in solution. ...
Ionic bonding
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
C2 Revision Quick Questions FT
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
C2 Revision Quick Questions FT
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
C2 revision slides V3 + questions + MS – F
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
C2 Additional Chemistry Thursday 14 May
... State that when chemical reactions occur, energy is transferred to or from the surroundings. State what an exothermic reaction is in terms of energy and give examples. Define an endothermic reaction in terms of energy and give examples. Recall that if a reversible reaction is exothermic in one direc ...
... State that when chemical reactions occur, energy is transferred to or from the surroundings. State what an exothermic reaction is in terms of energy and give examples. Define an endothermic reaction in terms of energy and give examples. Recall that if a reversible reaction is exothermic in one direc ...
Lecture Notes - Academic Home Page
... • Metals: readily form cations • Non-Metals: readily form anions ...
... • Metals: readily form cations • Non-Metals: readily form anions ...
Section 4.8: The Structure and Properties of Solids
... orthorhombic lattice structure of linear H–Cl molecules. 5. Doping means adding a small amount of an additional element, such as boron or arsenic, to a semiconductor. This is done to change the conductive properties of the ...
... orthorhombic lattice structure of linear H–Cl molecules. 5. Doping means adding a small amount of an additional element, such as boron or arsenic, to a semiconductor. This is done to change the conductive properties of the ...
4.5 Solid fast-ion conductors 1
... ex)on a rigid sphere model, the interstices between the anions(radius rA) in a face-centred cubic lattice would only allow penetration by a cation(0.15rA). however, in the case of I- ions(radius 220pm), interstices offer an aperture of only 34pm radius, the transport of Li+ (r3=55pm) is significant ...
... ex)on a rigid sphere model, the interstices between the anions(radius rA) in a face-centred cubic lattice would only allow penetration by a cation(0.15rA). however, in the case of I- ions(radius 220pm), interstices offer an aperture of only 34pm radius, the transport of Li+ (r3=55pm) is significant ...
CHEM1100 Practice Exam 2 You have 120 minutes to complete this
... You have 120 minutes to complete this exam. Answer all questions. To receive credit you must show your reasoning and all calculations in the bluebook. Report numerical answers with the correct number of significant figures and with correct units. No speaking is allowed during the exam. You must use ...
... You have 120 minutes to complete this exam. Answer all questions. To receive credit you must show your reasoning and all calculations in the bluebook. Report numerical answers with the correct number of significant figures and with correct units. No speaking is allowed during the exam. You must use ...
Chapter 7 Ionic and Metallic Bonding
... Section 7.2 Ionic Bonds and Ionic Compounds OBJECTIVES: ...
... Section 7.2 Ionic Bonds and Ionic Compounds OBJECTIVES: ...
Ch2hon ppt part 3
... (a)Because there are four chlorine atoms present, the compound is silicon tetrachloride. (b)There are four phosphorus atoms and ten oxygen atoms present, so the compound is tetraphosphorus decoxide. Note that the “a” is omitted in “deca.” ...
... (a)Because there are four chlorine atoms present, the compound is silicon tetrachloride. (b)There are four phosphorus atoms and ten oxygen atoms present, so the compound is tetraphosphorus decoxide. Note that the “a” is omitted in “deca.” ...
Name: Per: Date: Unit 1. Materials: Formulating Matter B. Periodic
... Use the two tables of common ions below. a. Potassium chloride is “lite salt”, used by many people with hypertension. b. CaSO4 is a component of plaster. c. A substance composed of Ca2+ and PO43- ions is found in some forms of phosphorus-containing fertilizer. This substance is also a major componen ...
... Use the two tables of common ions below. a. Potassium chloride is “lite salt”, used by many people with hypertension. b. CaSO4 is a component of plaster. c. A substance composed of Ca2+ and PO43- ions is found in some forms of phosphorus-containing fertilizer. This substance is also a major componen ...
Ionic compound

In chemistry, an ionic compound is a chemical compound in which ions are held together in a structure by electrostatic forces termed ionic bonds. The positively charged ions are called cations and the negatively charged ions are called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the carbonate ion (CO32−) in calcium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network, usually in a crystalline structure.Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids they are almost always electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.Ionic compounds without the acidic hydrogen ion (H+), or the basic ions hydroxide (OH−) or oxide (O2−), are also known as salts and can be formed by acid-base reactions. Ionic compounds containing hydrogen ions are classified as acids and compounds containing hydroxide or oxide ions are classified as bases.