* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download + H 2
Physical organic chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
History of electrochemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical bond wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Ionic liquid wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Acid–base reaction wikipedia , lookup
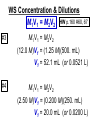
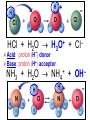
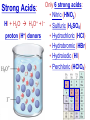
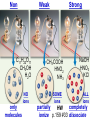
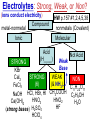
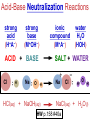
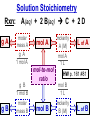
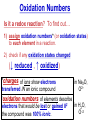
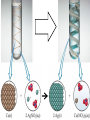
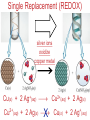
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Unit 4 (Chapter 4): Aqueous Reactions & Solution Stoichiometry John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall, Inc. Solutions: + • homogeneous mixtures: evenly mixed (same) • solvent is present in greatest abundance. • solute dissolved in/by solvent Molarity • Molarity (M) is a measure of the concentration of a solution. moles of solute (mol) Molarity (M) = liters of solution (L) units: mol/L or –1 ∙ mol L What’s the concentration of a solution with 29.2 g of sodium chloride in 250. mL of water? 29.2 g NaCl x 1 mol NaCl = 0.500 mol NaCl = 2.00 M 58.44 g NaCl NaCl 0.250 L Solution Prep from Solid 1-Calc & Mass solute WS #1-2 2-Add solvent, swirl to dissolve Conc. Calc’s 3-Fill with DI water to mark 4-Mix (Cap & Invert to mix evenly) WS Concentration & Dilutions #1 5.00 g NaHCO3 x 1 mol NaHCO3 x 1 L NaHCO3 = 84.01 g NaHCO3 0.100 mol NaHCO3 0.595 L NaHCO3 #2 1.20 mol CuSO4 159.62 g CuSO4 x 0.275 L CuSO4 x 1 L CuSO4 1 mol CuSO4 = 52.7 g CuSO4 Solution Prep by Dilution 1-Calc M1V1=M2V2 M1V1 = M2V2 2-Pipet V1 from concentrated WS #3-4 3-Fill to mark with DI water Dilutions 4-Mix (Cap & Invert to mix evenly) WS Concentration & Dilutions M1V1 = M2V2 HW p.160 #60, 67 #3 M1V1 = M2V2 (12.0 M)V1 = (1.25 M)(500. mL) V1 = 52.1 mL (or 0.0521 L) #4 M1V1 = M2V2 (2.50 M)V1 = (0.200 M)(250. mL) V1 = 20.0 mL (or 0.0200 L) H H H – O O Cl + H Cl H H HCl + H2O H3O+ + Cl– Acid: proton (H+) donor Base: proton (H+) acceptor NH3 + H2O NH4+ + OH– H H H N H O H H N H H – + H O H Strength of Acids and Bases STRONG: (complete ionization) (completely as ions) HA(aq) H+(aq) + A–(aq) MOH(aq) M+(aq) + OH–(aq) WEAK: (partial ionization) (mostly as molecules) HA(aq) + H2O(l) H3O+(aq) + A–(aq) B(aq) + H2O(l) BH+(aq) + OH–(aq) Only 6 strong acids: Strong Acids: • Nitric (HNO3) HI + H2O H3O+ + I– • Sulfuric (H SO ) 2 4 proton (H+) donors • Hydrochloric (HCl) • Hydrobromic (HBr) • Hydroiodic (HI) • Perchloric (HClO4) The strong bases are Strong Bases: soluble hydroxides –) of… (OH – + OH + H3O H2O + H2O • Group 1 (Li,Na,K) + proton (H ) acceptors • CBS (Ca, Ba, Sr) Mg(OH)2 & Be(OH)2 are not soluble Hydroxides of Group I and CBS ase Salts: Ionic Solids: (metal-nonmetal) dissociate (dissolve) by separation into ions Electrolytes: ions in solution that conduct electricity Non Weak C11H22O11 CH3OH H2O NO ions only molecules Strong CH3COOH HNO2 NH3 SOME ions NaOH HNO3 KCl ALL ions partially completely HW ionize p.159 #33 dissociate Electrolytes: Strong, Weak, or Non? (ions conduct electricity) metal-nonmetal Compound Ionic HW p.157 #1,2,4,5,38 nonmetals (Covalent) Molecular Acid (H____) Not Acid Weak STRONG Base KBr STRONG WEAK CaI2 NON (& NH3) (6) FeCl3 C11H22O11 NaOH HCl, HBr, HI CH3COOH C2H5OH HNO2 HNO3 Ca(OH)2 H2O HF (strong bases) H2SO4 HClO4 QUIZ!!! (at the bell) Electrolytes: Strong, Weak, or Non? metal-nonmetal Compound nonmetals (Covalent) Ionic STRONG Molecular Acid (H____) STRONG (6) Weak Base WEAK (& NH3) Not Acid NON Acid-Base Neutralization Reactions strong acid (H+A–) strong base (M+OH–) ionic compound (M+A–) ACID + BASE SALT + WATER + Cl H Na O water H 2O (HOH) H HCl(aq) + NaOH(aq) Na – Cl H O H NaCl(aq) + H2O(l) HW p.158 #40a Precipitation Reactions Double Replacement: 2 (aq) + (aq) 2 (precipitate) (aq) + precipitate: insoluble product (as predicted by solubility rules) Pb2+I– ( ) Solubility Rules ALWAYS Soluble ions: * Li+, Na+, K+, ... + NH * 4 * NO3– Group I (alkali metals) ammonium nitrate Common Precipitates form with: Ag+, Pb2+, Hg2+ (AP/H) OH– (hydroxide) CO32– (carbonate) WS Solubility & NIE’s #1 examples AgCl, PbI2 Cu(OH)2 CaCO3 Molecular Equation • reactants and products in molecular form AgNO3(aq) + KCl(aq) AgCl(s) + KNO3(aq) Ionic Equation • Strong Electrolytes are Dissociated as ions (strong acids, strong bases, soluble salts) Ag+(aq) + NO3–(aq) + K+(aq) + Cl–(aq) AgCl(s) + K+(aq) + NO3–(aq) Net Ionic Equation (NIE) • Cross out Spectator Ions (no change) (same state) (same charge) • only species left are those that react (change) during the course of the reaction. Ag+(aq) + NO3–(aq) + K+(aq) + Cl–(aq) Net NIE: AgCl(s) + K+(aq) + NO3–(aq) Ag+(aq) + Cl–(aq) AgCl(s) Balanced Net Ionic Equations comp – diss – cross – net – bal 1. Write a Complete molecular equation. 2. Dissociate all strong electrolytes(aq) . (solubility rules) 3. Cross out spectators (same charge & state) 4. Write the Net ionic equation with the species that remain. 5. Balance the NIE. Balanced Net Ionic Equations comp – diss – cross – net – bal + 2– 2+ – + – 1) (NH4)2SO4 + Ba(NO3)2 → BaSO4 + NH4NO3 Ba2+ + SO42– → BaSO4 + – 2+ – + – 2) NaOH + MgBr2 → NaBr + Mg(OH)2 Mg2+ + 2 OH– → Mg(OH)2(s) HW p.158 #21 Neutralization Reactions When a Strong Acid reacts with a Strong Base, the net ionic equation is… H+ + OH– H2O HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) H+ + Cl– + Na+ + OH– Na+ + Cl– + H2O Neutralization Reactions When a Weak acid reacts with a Strong base, the net ionic equation is… HX + OH– X– + H2O HF(aq) + KOH(aq) KF(aq) + H2O(l) (dissociate ONLY STRONG electrolytes) HF + Na+ + OH– Na+ + F– + H2O HW p.159 #40 (finish) Balanced Net Ionic Equations comp – diss – cross – net – bal + 2– 2+ – + – (NH4)2SO4 + Ba(NO3)2 → BaSO4 + NH4NO3 Ba2+ + SO42– → BaSO4(s) + – + – HF(aq) + KOH(aq) KF(aq) + H2O(l) HF + OH– F– + H2O WS Solubility & NIE’s #2 Gas-Forming Reactions H2 Demo (M0) (H+) Single Rep: Metal + Acid + 2– Ex: Zn(s) + H2SO4(aq) NIE: Zn(s) + 2 H+(aq) (M+) (gas) Metal Ion + H2 2+ 2– ZnSO4(aq) + H2(g) Zn2+(aq) + H2(g) (gas) H2O(l) + CO2(g) (CO32–) CO2 Demo (H+) Double Rep: Acid + Carbonate Salt + H2CO3(aq) (or Bicarbonate) (decomposes HW p. 159 #43 (HCO3–) immediately) Ex: HCl(aq) + CaCO3(s) CaCl2(aq) + H2O(l) + CO2(g) NIE: 2 H+(aq) + CaCO3(s) Ca2+(aq) + H2O(l) + CO2(g) CH3COOH + NaHCO3 CH3COONa + H2O + CO2 Solution Stoichiometry Rxn: gA A(aq) + 2 B(aq) C + 2 D molar mass A g A 1 mol A mol A L of A mol A 1L mol-to-mol ratio gB molarity A (M) HW p. 161 #81 g B 1 mol B mol B 1L molar mass B molarity B (M) mol B L of B Oxidation-Reduction Reactions (REDOX) video clip (One cannot occur without the other) LEO says GER Oxidation Numbers Is it a redox reaction? To find out… 1) assign oxidation numbers* (or oxidation states) to each element in a reaction. 2) check if any oxidation states changed (↓ reduced , ↑ oxidized) *charges of ions show electrons transferred IN an ionic compound in Na2O, O2– *oxidation numbers of elements describe electrons that would be lost or gained IF the compound was 100% ionic. in H2O, O–2 Assigning Oxidation Numbers 1. All pure elements are 0 2. Monatomic ion is its charge (Mg2+ has +2) 3. Most nonmetals tend to be negative, but some are positive in certain compounds or ions. (in SO3 , O is –2 but S is +6) O is −2 always but in peroxide ion is −1 (O22–) H is +1 with nonmetals, −1 with metals F is always −1. other halogens are −1, BUT can be positive, like in oxyanions. Ex. ClO3– or NO3– or SO42– Oxidation Numbers • The sum of the ox. #’s in a neutral compound is 0. • The sum of the ox. #’s in a polyatomic ion is the charge on the ion. Determine the oxidation number of: Sulfur in… SO2 Chromium in… K2Cr2O7 Nitrogen in… NH4+ Cobalt in… [CoCl6]3– Classifying REDOX Reactions All rxns (but…NOT double replacement) Synthesis A + B → AB (0 0 → +/–) Decomposition 2→1 AB → A + B (+/– → 0 0) 1→2 Single Replacement Combustion AB + C → A + CB (+/– 0 → 0 +/–) CxHy + O2 → CO2 + H2O (–/+ 0 → +/– +/–) Single Replacement (REDOX) silver ions oxidize copper metal Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s) +(aq) Cu2+(aq) + 2 Ag(s) Cu (s) + 2 Ag X Cannot displace H+ from acid to make H2(g) increasing ease of oxidation Activity Series of Metals Writing REDOX Reactions Write the net ionic equation for the reaction of solid zinc in a solution of hydrochloric acid. comp – diss – cross – net – bal 0 +1 –1 +2 –1 0 Mg(s) + HCl(aq) MgCl2(aq) + H2(g) Mg + 2 H+ Mg+2 + H2 Classify the reaction in two ways. Single-Replacement and Redox ox Mg + 2 H+ Mg2+ + H2(g) red What is red & what is ox? WS Aq Soln’s & Chem Rxns