IPC Semester Exam Review – Chemistry Topics
... Questions will include multiple-choice and matching. You will need a calculator and a pencil for the Scantron form. A periodic table and conversion chart will be provided. The Nature of Science Identify each of the following examples as PURE or APPLIED sciences. 1. Development of the computer ch ...
... Questions will include multiple-choice and matching. You will need a calculator and a pencil for the Scantron form. A periodic table and conversion chart will be provided. The Nature of Science Identify each of the following examples as PURE or APPLIED sciences. 1. Development of the computer ch ...
7.1: “Avogadro`s Number and Molar Conversions” Day 1 Atoms
... 4. Conversion Factors are Equivalent to _________. a. 6.022 X 1023 particles = ____________________________ b. From this definition, we get 2 conversion factors: ...
... 4. Conversion Factors are Equivalent to _________. a. 6.022 X 1023 particles = ____________________________ b. From this definition, we get 2 conversion factors: ...
Physical Chemistry of Colloids and Surfaces – Final Exam Review 4-30-02
... -H132 is negative when the Hamaker constant of the intermediate material is between that of the other two. -When interacting over long ranges, van der Waals interactions are weakened by retardation effects due to equivalent time scales for electron density fluctuations and the time for EM radiation ...
... -H132 is negative when the Hamaker constant of the intermediate material is between that of the other two. -When interacting over long ranges, van der Waals interactions are weakened by retardation effects due to equivalent time scales for electron density fluctuations and the time for EM radiation ...
8th Grade: First Semester Final Review
... 25. Deposition is a change from a _____. a. gas to a solid b. liquid to a gas 26. Vaporization that occurs within a liquid is _____. a. boiling b. evaporation 27. Deposition could be best described as the opposite of _____. a. condensation b. sublimation 28. What happens when temperature increases o ...
... 25. Deposition is a change from a _____. a. gas to a solid b. liquid to a gas 26. Vaporization that occurs within a liquid is _____. a. boiling b. evaporation 27. Deposition could be best described as the opposite of _____. a. condensation b. sublimation 28. What happens when temperature increases o ...
Combustion and Nuclear Reactions
... If you shake a rope through a fence with vertical slats, only waves that vibrate up and down will pass through If you shake the rope side to side, the waves will be blocked A polarizing light filter acts like the slats in a fence. ▪ It only allows waves that vibrate in one direction to pass th ...
... If you shake a rope through a fence with vertical slats, only waves that vibrate up and down will pass through If you shake the rope side to side, the waves will be blocked A polarizing light filter acts like the slats in a fence. ▪ It only allows waves that vibrate in one direction to pass th ...
Size-Resolved Kinetic Measurements of Aluminum Nanoparticle
... in the number of atoms among different materials is ∼30%, allowing only ∼10% variation in particle size. This implies that the number of atoms present in the particle can be used to determine particle size independent of particle composition and shape. Using the DMA-generated monodisperse aerosols ( ...
... in the number of atoms among different materials is ∼30%, allowing only ∼10% variation in particle size. This implies that the number of atoms present in the particle can be used to determine particle size independent of particle composition and shape. Using the DMA-generated monodisperse aerosols ( ...
Water Pollution
... Large coal-fired power plants lacking ash-control equipment can introduce up to several hundred milliCuries of radionuclides into the atmosphere each year, far more than either an equivalent nuclear or oil-fired power plant. ...
... Large coal-fired power plants lacking ash-control equipment can introduce up to several hundred milliCuries of radionuclides into the atmosphere each year, far more than either an equivalent nuclear or oil-fired power plant. ...
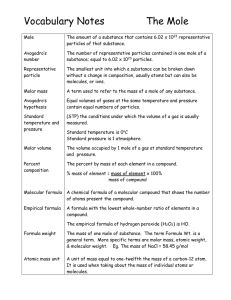
Vocabulary Notes
... The mass of one mole of substance. The term Formula Wt. is a general term. More specific terms are molar mass, atomic weight, & molecular weight. Eg. The mass of NaCl = 58.45 g/mol ...
... The mass of one mole of substance. The term Formula Wt. is a general term. More specific terms are molar mass, atomic weight, & molecular weight. Eg. The mass of NaCl = 58.45 g/mol ...
Chemical laboratories Dipl.-Ing.(FH) Giovanna
... Automated Determination of ammonium, phosphate, nitrate and nitrite by flow injection analysis (FIA) ...
... Automated Determination of ammonium, phosphate, nitrate and nitrite by flow injection analysis (FIA) ...
Final Exam Review Guide
... Translation (moving from place to place) – Liquid and Gas only Describe the “Kinetic Theory of Gases” and list the three assumptions associated with it. What volume does one mole of any gas occupy at STP? 22.4 L Kinetic theory states that all matter is composed of particles and the particles are in ...
... Translation (moving from place to place) – Liquid and Gas only Describe the “Kinetic Theory of Gases” and list the three assumptions associated with it. What volume does one mole of any gas occupy at STP? 22.4 L Kinetic theory states that all matter is composed of particles and the particles are in ...
Matter
... dilute solution. A solution that has as much solute as it can hold is called a saturated solution. Solutes can be solids, liquids, or gases. A suspension is a kind of mixture that separates if it is left alone for some time. One factor that makes suspensions different from solutions is the size of t ...
... dilute solution. A solution that has as much solute as it can hold is called a saturated solution. Solutes can be solids, liquids, or gases. A suspension is a kind of mixture that separates if it is left alone for some time. One factor that makes suspensions different from solutions is the size of t ...
MatterPP4
... The solubility of a solute is the amount of solute needed to make a saturated solution using a given amount of solvent at a certain temperature. Solubility is usually expressed in grams of solute per 100 ml of solvent (g/100ml) Three (3) methods that affect solubility ...
... The solubility of a solute is the amount of solute needed to make a saturated solution using a given amount of solvent at a certain temperature. Solubility is usually expressed in grams of solute per 100 ml of solvent (g/100ml) Three (3) methods that affect solubility ...
Resumen Science I Trimestre II Parcial Definitions: Element: pure
... visible. Ex. Pizza, oil and water, gallo pinto. Homogeneous mixtures: same composition through any one region of a mixture has the same ratio of a substance as any other region, compounds can’t be seen as individual identifiable entities mixed as much finer level, not readily distinguish. Ex. Water ...
... visible. Ex. Pizza, oil and water, gallo pinto. Homogeneous mixtures: same composition through any one region of a mixture has the same ratio of a substance as any other region, compounds can’t be seen as individual identifiable entities mixed as much finer level, not readily distinguish. Ex. Water ...
Glossary
... The lifetime of a species whose concentration is changing is the time required for the concentration to fall to 1/e of its initial value. Lifetimes in chemistry vary over a large range, from around 10-16 s for some very short-lived atomic states to billions of years for elements that are weakly radi ...
... The lifetime of a species whose concentration is changing is the time required for the concentration to fall to 1/e of its initial value. Lifetimes in chemistry vary over a large range, from around 10-16 s for some very short-lived atomic states to billions of years for elements that are weakly radi ...
Chemistry Midterm Review
... 1) ___________ which has a definite shape and definite volume. 2) __________ which has an indefinite shape but definite volume 3) __________ which has an indefinite shape and indefinite volume Matter can be classified as either: - ________ _________ which cannot be separated into simpler substances ...
... 1) ___________ which has a definite shape and definite volume. 2) __________ which has an indefinite shape but definite volume 3) __________ which has an indefinite shape and indefinite volume Matter can be classified as either: - ________ _________ which cannot be separated into simpler substances ...
Scientific Notation - Warren County Public Schools
... What subatomic particle has the same value with each isotope of carbon above? What subatomic particle has different values with each isotope of carbon above? ...
... What subatomic particle has the same value with each isotope of carbon above? What subatomic particle has different values with each isotope of carbon above? ...
Chemistry Vocab for Quiz 12/21 or 12/22 Atom – The smallest
... Physical change – A change that alters the form or appearance of a material but does not make the material into a different substance. Chemical change – A change in matter that produces a new substance. Solution – A well mixed mixture. Solubility – A measure of how well a solute can be dissolved at ...
... Physical change – A change that alters the form or appearance of a material but does not make the material into a different substance. Chemical change – A change in matter that produces a new substance. Solution – A well mixed mixture. Solubility – A measure of how well a solute can be dissolved at ...
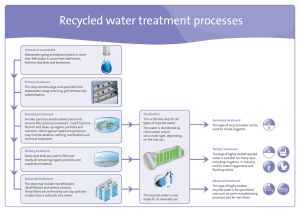
Recycled water treatment processes
... This is the last step for all types of recycled water. The water is disinfected by chlorination and/or ultra violet light, depending on the end use. ...
... This is the last step for all types of recycled water. The water is disinfected by chlorination and/or ultra violet light, depending on the end use. ...
Document
... Generally, three tests or more if time permits should be performed by the student. Test results should be plus or minus 0.5 pH units. The goal is to obtain data that is accurate and precise. Any result that is extreme should be questioned and with your classroom mentor or FRWC staff. The sample mode ...
... Generally, three tests or more if time permits should be performed by the student. Test results should be plus or minus 0.5 pH units. The goal is to obtain data that is accurate and precise. Any result that is extreme should be questioned and with your classroom mentor or FRWC staff. The sample mode ...
Concept Scalewatcher
... 'scrubbing' or cleaning, or to achieve a chemical/mineral effect as part of the production process, so increasing the scaling tendency. Scaling deposits are very common in flow lines subject to changes in pressure or temperature. Regardless of how hard water effects are achieved, the outcome is the ...
... 'scrubbing' or cleaning, or to achieve a chemical/mineral effect as part of the production process, so increasing the scaling tendency. Scaling deposits are very common in flow lines subject to changes in pressure or temperature. Regardless of how hard water effects are achieved, the outcome is the ...
Jeopardy
... If an atom has 7 electrons, how many electrons will be on the outer most energy level? ...
... If an atom has 7 electrons, how many electrons will be on the outer most energy level? ...
CHAPTER 11 – NUCLEAR CHEMISTRY
... - provide energy Ex: Energy from fission reactions is used for electric power Ex: Fusion reactions take place in the sun and produce solar energy Risks: - wastes from nuclear reactors are very radioactive - waste must be stored for more than 100 thousand years without leaking into the environment - ...
... - provide energy Ex: Energy from fission reactions is used for electric power Ex: Fusion reactions take place in the sun and produce solar energy Risks: - wastes from nuclear reactors are very radioactive - waste must be stored for more than 100 thousand years without leaking into the environment - ...
Particle-size distribution
The particle-size distribution (PSD) of a powder, or granular material, or particles dispersed in fluid, is a list of values or a mathematical function that defines the relative amount, typically by mass, of particles present according to size. PSD is also known as grain size distribution.