CHAPTER 3 Atoms: The Building Blocks of Matter
... – All matter is composed of extremely small particles called atoms – Atoms of a given element are identical in size, mass, and other properties – Atoms cannot be subdivided, created, or destroyed – Atoms of different elements combine in simple whole number ratios to form compounds – In chemical reac ...
... – All matter is composed of extremely small particles called atoms – Atoms of a given element are identical in size, mass, and other properties – Atoms cannot be subdivided, created, or destroyed – Atoms of different elements combine in simple whole number ratios to form compounds – In chemical reac ...
Instrumental Analysis as Applied to Architectural Materials
... Energy-dispersive X-ray spectroscopy (EDS or EDX) identifies concentrations of elements of less than 0.1 percent. Today, most SEMs are equipped with EDS. The interaction of the SEM electrons with sample material produces X-rays that are characteristic of specific elements. As a result, EDS provides ...
... Energy-dispersive X-ray spectroscopy (EDS or EDX) identifies concentrations of elements of less than 0.1 percent. Today, most SEMs are equipped with EDS. The interaction of the SEM electrons with sample material produces X-rays that are characteristic of specific elements. As a result, EDS provides ...
TANNIC ACID
... - Sample heating temp.: 80o - Sample heating time: 40 min - Syringe temperature: 85o - Sample gas injection: 0.4 ml Calculation ...
... - Sample heating temp.: 80o - Sample heating time: 40 min - Syringe temperature: 85o - Sample gas injection: 0.4 ml Calculation ...
terpconnect.umd.edu
... Rayleigh limit: the equilibrium state at which further addition of charge will cause the drop to become unstable and break ...
... Rayleigh limit: the equilibrium state at which further addition of charge will cause the drop to become unstable and break ...
Chapter 1
... A gas at 25ºC fills a container whose volume is 1.05 x 103 cm3. The container plus the gas have a mass of 837.6 g. The container when emptied of all gas has a mass of 836.2 g. What is the density of the gas at 25ºC? To how many significant figures should the mass of the container be measured (with a ...
... A gas at 25ºC fills a container whose volume is 1.05 x 103 cm3. The container plus the gas have a mass of 837.6 g. The container when emptied of all gas has a mass of 836.2 g. What is the density of the gas at 25ºC? To how many significant figures should the mass of the container be measured (with a ...
Here is the Original File
... 0.33 mL/second while being stirred rapidly. This is done in order to control the particle size of the colloid. The black sludge product was centrifuged for 1 min at 1000 rpm. The sample was decanted of ammonia and the tetramethylammonium hydroxide was added. The sample was isolated and the magnetic ...
... 0.33 mL/second while being stirred rapidly. This is done in order to control the particle size of the colloid. The black sludge product was centrifuged for 1 min at 1000 rpm. The sample was decanted of ammonia and the tetramethylammonium hydroxide was added. The sample was isolated and the magnetic ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... and nitrogen [N]; for teeth & BONES = calcium [Ca] and phosphorus [P]; for taste buds = zinc [Zn]; for nervous system = copper [Cu]; for blood = iron [Fe] *e. There are currently 118 known ELEMENTS and 92 are found in nature, while the others are SYNTHETICALLY (man-made), but we only use between 30- ...
... and nitrogen [N]; for teeth & BONES = calcium [Ca] and phosphorus [P]; for taste buds = zinc [Zn]; for nervous system = copper [Cu]; for blood = iron [Fe] *e. There are currently 118 known ELEMENTS and 92 are found in nature, while the others are SYNTHETICALLY (man-made), but we only use between 30- ...
Atomic arrangement, short and long range order, point. Direction
... longrange order. In an ideal gas the arrangementof an atom at any point in space is independent of the arrangement of other atoms. Thus, bothlong-range and shortrange order are absent in the ideal gas, but liquids and amorphous solidsexhibit sho rt-rangeordera certain regularity in the arrangement o ...
... longrange order. In an ideal gas the arrangementof an atom at any point in space is independent of the arrangement of other atoms. Thus, bothlong-range and shortrange order are absent in the ideal gas, but liquids and amorphous solidsexhibit sho rt-rangeordera certain regularity in the arrangement o ...
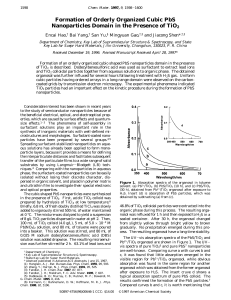
Formation of Orderly Organized Cubic PbS Nanoparticles Domain in
... organic phase during this process. The resulting organosol was refluxed for 1 h and then exposed to H2S in a sealed container. After 30 h, the organosol changed from slightly yellow through bright yellow to brown gradually. No precipitation emerged during this process. The resulting organosol have a ...
... organic phase during this process. The resulting organosol was refluxed for 1 h and then exposed to H2S in a sealed container. After 30 h, the organosol changed from slightly yellow through bright yellow to brown gradually. No precipitation emerged during this process. The resulting organosol have a ...
CHEM 101 1st Major (Term 161)
... 1. A weather balloon filled with helium has a diameter of 3.50 ft. What is the mass in grams of the helium in the balloon? The density of the helium is 0.166 g/L. The volume can be calculated as (4/3)r3. [1 ft =12 in; 1 in = 2.54 cm] A) 106 g B) 271 g C) 21.3 g D) 79.9 g E) 63.6 g ...
... 1. A weather balloon filled with helium has a diameter of 3.50 ft. What is the mass in grams of the helium in the balloon? The density of the helium is 0.166 g/L. The volume can be calculated as (4/3)r3. [1 ft =12 in; 1 in = 2.54 cm] A) 106 g B) 271 g C) 21.3 g D) 79.9 g E) 63.6 g ...
Nuclear Astrophysics (1)
... The chemical potential obtained from the total number density n provides information on energy/momentum distributions of particles. It is only determined up to a constant. If energy generation due to mass differences in reactions is involved, the above equation is correct, if ...
... The chemical potential obtained from the total number density n provides information on energy/momentum distributions of particles. It is only determined up to a constant. If energy generation due to mass differences in reactions is involved, the above equation is correct, if ...
PowerPoint material for lecture 1 (September 4, 2012)
... • In bulk solids, all molecules are surrounded by and bound to neighboring atoms and the forces are in balance. Surface atoms are bound only on one side, leaving unbalanced atomic and molecular forces on the surface. These forces attract gases and molecules Van der Waals force, physical adsorpti ...
... • In bulk solids, all molecules are surrounded by and bound to neighboring atoms and the forces are in balance. Surface atoms are bound only on one side, leaving unbalanced atomic and molecular forces on the surface. These forces attract gases and molecules Van der Waals force, physical adsorpti ...
ELAB: One of the Most Potent Amino Acid Analysis
... TMS esters of carboxylic acids) can be decomposed by active sites in the chromatographic system. Enantiomer-labeling [5] If the losses mentioned above are reproducible, then they may be calculated and taken into account in the result. Generally this is not the case, especially when components from a ...
... TMS esters of carboxylic acids) can be decomposed by active sites in the chromatographic system. Enantiomer-labeling [5] If the losses mentioned above are reproducible, then they may be calculated and taken into account in the result. Generally this is not the case, especially when components from a ...
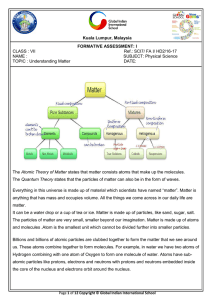
Chapter 15- Classification of Matter
... a. __________________ - either an element or a compound. i. When all the atoms in a substance are alike, the substance in an ________________. ii. A ___________________ is a substance with two or more elements combined in a fixed proportion. b. Two or more substances that can be easily separated by ...
... a. __________________ - either an element or a compound. i. When all the atoms in a substance are alike, the substance in an ________________. ii. A ___________________ is a substance with two or more elements combined in a fixed proportion. b. Two or more substances that can be easily separated by ...
Introduction to colloid and sol-gel chemistry
... down under gravity. Colloids can pass through ordinary filter paper but do not pass through animal membranes, e.g. a colloidal solution of gold. Total interface area of the colloidal dispersed particles is very large due to their submicroscopic size. The huge area-to-volume ratio determines specific ...
... down under gravity. Colloids can pass through ordinary filter paper but do not pass through animal membranes, e.g. a colloidal solution of gold. Total interface area of the colloidal dispersed particles is very large due to their submicroscopic size. The huge area-to-volume ratio determines specific ...
A SWDY OF MErnANISM OF RADIATIOO IN LlMINOOS FLAMES
... and a '- is related to C• .'!here is no satisfactory method for calculating C, . it can be measured by instrument, and it is the key problem that puzzled one who wants to investigate the mechanism of luminous flame . 3. The main factors which affect the emissivity of luminous f'Iames are the emissiv ...
... and a '- is related to C• .'!here is no satisfactory method for calculating C, . it can be measured by instrument, and it is the key problem that puzzled one who wants to investigate the mechanism of luminous flame . 3. The main factors which affect the emissivity of luminous f'Iames are the emissiv ...
Name Objective 1: Matter and Energy C3H8 + 5O2 → 3CO2 + 4H2O
... 23. Which of the following tables would be used to correctly describe the difference between metals and non-metals? (6.6A) ...
... 23. Which of the following tables would be used to correctly describe the difference between metals and non-metals? (6.6A) ...
Unit 3: Properties and States of Matter
... • You and your lab partner must go around to each station and identify whether the property being demonstrated is a physical or chemical property and state why. ...
... • You and your lab partner must go around to each station and identify whether the property being demonstrated is a physical or chemical property and state why. ...
AP Exam One Retake Qualifying Assignment
... takes the shape and volume of its container gaseous state of matter at a temperature less than its boiling point rusting of metal NaCl in the reaction between sodium metal and chlorine gas ...
... takes the shape and volume of its container gaseous state of matter at a temperature less than its boiling point rusting of metal NaCl in the reaction between sodium metal and chlorine gas ...
File
... the mass of the products equals 80g (Law of conservation of mass). You should also notice that in CH4 there is one Carbon atom, and four hydrogen atoms (Law of definite proportions). Electrolysis Reactions: Carried out in a Hoffman’s apparatus (shown to the right), it splits water compounds into o ...
... the mass of the products equals 80g (Law of conservation of mass). You should also notice that in CH4 there is one Carbon atom, and four hydrogen atoms (Law of definite proportions). Electrolysis Reactions: Carried out in a Hoffman’s apparatus (shown to the right), it splits water compounds into o ...
Measuring Matter - eChem2Bper2Miller
... number of grams of each element in one mole of the compound. Then add the masses of the elements in the Compound. ...
... number of grams of each element in one mole of the compound. Then add the masses of the elements in the Compound. ...
Chapter 3 Discovering the atom and subatomic particles (History of
... invented precise scale to weigh small amounts of matter accurate to 0.0005 grams, about 1/100 of a drop of water mass of all reactants = mass of all products cannot create matter from nothing, can only change form matter is not created from nothing, only changes form during chemical reactions ...
... invented precise scale to weigh small amounts of matter accurate to 0.0005 grams, about 1/100 of a drop of water mass of all reactants = mass of all products cannot create matter from nothing, can only change form matter is not created from nothing, only changes form during chemical reactions ...
Chapter 3 Discovering the atom and subatomic particles (History of
... invented precise scale to weigh small amounts of matter accurate to 0.0005 grams, about 1/100 of a drop of water mass of all reactants = mass of all products cannot create matter from nothing, can only change form matter is not created from nothing, only changes form during chemical reactions ...
... invented precise scale to weigh small amounts of matter accurate to 0.0005 grams, about 1/100 of a drop of water mass of all reactants = mass of all products cannot create matter from nothing, can only change form matter is not created from nothing, only changes form during chemical reactions ...
worksheer format 11-12
... another substance. They appear very similar to solutions, but the particles are suspended in the solution rather than fully dissolved. The difference between a colloid and a suspension is that the particles will not settle to the bottom over a period of time, they will stay suspended or float. An ex ...
... another substance. They appear very similar to solutions, but the particles are suspended in the solution rather than fully dissolved. The difference between a colloid and a suspension is that the particles will not settle to the bottom over a period of time, they will stay suspended or float. An ex ...
Particle-size distribution
The particle-size distribution (PSD) of a powder, or granular material, or particles dispersed in fluid, is a list of values or a mathematical function that defines the relative amount, typically by mass, of particles present according to size. PSD is also known as grain size distribution.