Lesson 2: Magnetism
... Compass and dip needles were used to find magnetite in Sweden in the Middle Ages, making the magnetic method the oldest of all applied geophysical techniques. This method of magnetic prospecting for ores ...
... Compass and dip needles were used to find magnetite in Sweden in the Middle Ages, making the magnetic method the oldest of all applied geophysical techniques. This method of magnetic prospecting for ores ...
Slide 1
... run from left to right: g= 0, 1 or 2 For a broad range of parameters, there is only one running solution, and then the electrons are fully polarized! ...
... run from left to right: g= 0, 1 or 2 For a broad range of parameters, there is only one running solution, and then the electrons are fully polarized! ...
Lecture 5
... • minimized when M is parallel to long axis • uniaxial anisotropy energy density constant: ...
... • minimized when M is parallel to long axis • uniaxial anisotropy energy density constant: ...
Supplementary Information (doc 3822K)
... and M vs. H curves in magnetic field sweep rates 10 Oe/sec and 50 Oe/sec. (the corresponding M vs. H curves at 100 Oe/sec are shown in Figure 2.b of the article). No change was observed at all temperatures, in both the AC susceptibility and DC magnetization by varying the magnetic field sweep rate. ...
... and M vs. H curves in magnetic field sweep rates 10 Oe/sec and 50 Oe/sec. (the corresponding M vs. H curves at 100 Oe/sec are shown in Figure 2.b of the article). No change was observed at all temperatures, in both the AC susceptibility and DC magnetization by varying the magnetic field sweep rate. ...
An Introduction to NMR Spectroscopy
... exactly equal to the energy separation between the states (E). This amount of energy is usually found in the radiofrequency range. The condition for absorption of energy is called the condition of resonance. It can be calculated as the following: ...
... exactly equal to the energy separation between the states (E). This amount of energy is usually found in the radiofrequency range. The condition for absorption of energy is called the condition of resonance. It can be calculated as the following: ...
CHEM763 Special Topics for 2016 - UKZN Chemistry and Physics
... products, i.e. compounds that are produced by living organisms such as plants and microorganisms. In the course, you will be introduced to the different steps in the drug discovery pipeline and terms such as hit compound, a lead ...
... products, i.e. compounds that are produced by living organisms such as plants and microorganisms. In the course, you will be introduced to the different steps in the drug discovery pipeline and terms such as hit compound, a lead ...
Microstructured Resonators for Electron Spin Resonance
... Most implementations of electron paramagnetic resonance (EPR) use resonant structures for efficiently converting microwave signals to oscillating magnetic fields that excite transitions between electronic spin states. The same structures are also used to convert precessing magnetization into microwave ...
... Most implementations of electron paramagnetic resonance (EPR) use resonant structures for efficiently converting microwave signals to oscillating magnetic fields that excite transitions between electronic spin states. The same structures are also used to convert precessing magnetization into microwave ...
Full Text PDF
... if only the state of N d-electrons and one band electron is energetically stable. Consequently, for these materials, in contrast to the situation in DMS doped with Mn 2 + ions, the effective p— d interaction induced by the kinetic exchange should have a ferromagnetic character. To estimate the value ...
... if only the state of N d-electrons and one band electron is energetically stable. Consequently, for these materials, in contrast to the situation in DMS doped with Mn 2 + ions, the effective p— d interaction induced by the kinetic exchange should have a ferromagnetic character. To estimate the value ...
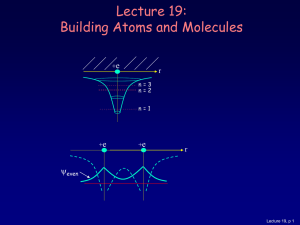
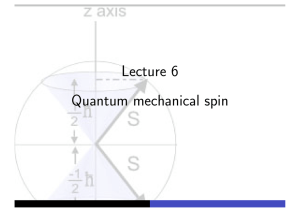
Quantum mechanical spin - Theory of Condensed Matter
... In addition to orbital angular momentum, L̂, quantum particles possess an intrinsic angular momentum known as spin, Ŝ. For fermions, spin is half-integer while, for bosons, it is integer. Wavefunction of electron expressed as a two-component spinor, ...
... In addition to orbital angular momentum, L̂, quantum particles possess an intrinsic angular momentum known as spin, Ŝ. For fermions, spin is half-integer while, for bosons, it is integer. Wavefunction of electron expressed as a two-component spinor, ...
Practice Exam III
... 1. Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the prod ...
... 1. Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the prod ...
doc
... interaction between the magnetic moment of the electron spin with a superimposed direct or alternating magnetic field. A ball with a central rod magnet, which rotates with low friction on an air cushion, acts as model electron. Two pairs of coils generate a constant magnetic field Bo and an alternat ...
... interaction between the magnetic moment of the electron spin with a superimposed direct or alternating magnetic field. A ball with a central rod magnet, which rotates with low friction on an air cushion, acts as model electron. Two pairs of coils generate a constant magnetic field Bo and an alternat ...
Computational Spectroscopy
... Transitions between electronic states are accompanied by vibrational and rotational transitions. Visible and ultraviolet regions ...
... Transitions between electronic states are accompanied by vibrational and rotational transitions. Visible and ultraviolet regions ...
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a technique for studying materials with unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of the spins of atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes or organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.