STUDY SHEET EXAM 2
... Define and/or describe: nucleus, isotope, valence shell, valence electron, atomic number, mass number, atomic mass, ion, cation, anion, monatomic ion, polyatomic ion, ionic bond, covalent bond, molecule, formula unit, mole cular mass, formula mass, mole, molar mass, empirical formula, molecular form ...
... Define and/or describe: nucleus, isotope, valence shell, valence electron, atomic number, mass number, atomic mass, ion, cation, anion, monatomic ion, polyatomic ion, ionic bond, covalent bond, molecule, formula unit, mole cular mass, formula mass, mole, molar mass, empirical formula, molecular form ...
MRI. Thermography. - med.muni
... operational costs is obtained with magnets having superconducting coil windings, which can produce fields of up to B = 10 T today, but must be cooled by liquid helium. Typical values of B used in practice are 1 – 3 T. Gradients (about several mT.m-1) of magnetic field are formed by additional coil ...
... operational costs is obtained with magnets having superconducting coil windings, which can produce fields of up to B = 10 T today, but must be cooled by liquid helium. Typical values of B used in practice are 1 – 3 T. Gradients (about several mT.m-1) of magnetic field are formed by additional coil ...
Einstein-Podolsky-Rosen paradox without entanglement
... coupling which produced the entanglement. Let us now turn to the effect of Bennett et al. [1] where entanglement was not used, and try to find why could we say about nonlocality in this case. The scheme is the following. There are three parties: Alice, Bob and Charlie. Alice and Bob are distantly loca ...
... coupling which produced the entanglement. Let us now turn to the effect of Bennett et al. [1] where entanglement was not used, and try to find why could we say about nonlocality in this case. The scheme is the following. There are three parties: Alice, Bob and Charlie. Alice and Bob are distantly loca ...
INFORMATION ON MASTER`S THESIS 1. Full name: VU THI MINH
... 11. Summary of the finding of the thesis: Thesis: “Anomalous magnetic moment of electrons and methods Pauli – Villars in quantum field theory” study the anomalous magnetic moment of the electron in quantum field theory. The main complementary magnetic moment based on perturbation theory via covarian ...
... 11. Summary of the finding of the thesis: Thesis: “Anomalous magnetic moment of electrons and methods Pauli – Villars in quantum field theory” study the anomalous magnetic moment of the electron in quantum field theory. The main complementary magnetic moment based on perturbation theory via covarian ...
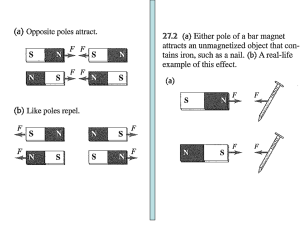
Permanent magnets are just collections of little current loops
... The unit of the magnetic field B (the Tesla) A] is the same as the electric field times a velocity B] is the same as the electric field divided by a velocity C] cannot be expressed as either of these ...
... The unit of the magnetic field B (the Tesla) A] is the same as the electric field times a velocity B] is the same as the electric field divided by a velocity C] cannot be expressed as either of these ...
Lecture 19: Magnetic properties and the Nephelauxetic effect
... The value of λ is negligible for very light atoms, but increases with increasing atomic weight, so that for heavier d-block elements, and for f-block elements, the orbital contribution is considerable. For 2nd and 3rd row dblock elements, λ is an order of magnitude larger than for the first-row anal ...
... The value of λ is negligible for very light atoms, but increases with increasing atomic weight, so that for heavier d-block elements, and for f-block elements, the orbital contribution is considerable. For 2nd and 3rd row dblock elements, λ is an order of magnitude larger than for the first-row anal ...
Nugget
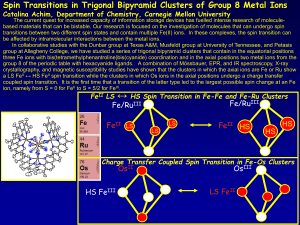
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
Lectures 5-6: Magnetic dipole moments
... Conclusion of Stern-Gerlach experiment: o With field on, classically expect random distribution at target. In fact find two bands as beam is split in two. o There is directional quantisation, parallel or antiparallel to B. o Atomic magnetic moment has z = ±B. o Find same deflection for all atoms w ...
... Conclusion of Stern-Gerlach experiment: o With field on, classically expect random distribution at target. In fact find two bands as beam is split in two. o There is directional quantisation, parallel or antiparallel to B. o Atomic magnetic moment has z = ±B. o Find same deflection for all atoms w ...
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a technique for studying materials with unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of the spins of atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes or organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.