Chemistry I Objectives Chapter 10
... number of particles from moles or the number of moles from particles. • III. Define and be able to calculate molar mass of an element and formula mass of a compound (gram atomic/formula mass). Use this to calculate moles from mass or mass from moles. • IV. Use molar volume (22.4 dm3) to calculate th ...
... number of particles from moles or the number of moles from particles. • III. Define and be able to calculate molar mass of an element and formula mass of a compound (gram atomic/formula mass). Use this to calculate moles from mass or mass from moles. • IV. Use molar volume (22.4 dm3) to calculate th ...
Mineral Chemistry Calculations
... create a series of formulas, one of which will be the correct chemical formula for the mineral • start with the formula with the yH2O, create a new formula by subtracting 1 H2O and 1 from the subscript associated with the non water oxygen in the formula • next add 2 (OH) waters = (OH)2 to the formul ...
... create a series of formulas, one of which will be the correct chemical formula for the mineral • start with the formula with the yH2O, create a new formula by subtracting 1 H2O and 1 from the subscript associated with the non water oxygen in the formula • next add 2 (OH) waters = (OH)2 to the formul ...
Quantum Mechanical Derivation of the Wallis Formula for $\ pi$
... A famous pre-Newtonian formula for π is obtained directly from the variational approach to the spectrum of the hydrogen atom in spaces of arbitrary dimensions greater than one, including the physical three dimensions. ...
... A famous pre-Newtonian formula for π is obtained directly from the variational approach to the spectrum of the hydrogen atom in spaces of arbitrary dimensions greater than one, including the physical three dimensions. ...
Ex5
... 1. For a single quantum particle of mass m, spectra p2/2m in a volume V the partition fundtion is Z1(m)=gV/3 with h / 2mk BT . The particle has a spin degeneracy g (g=2s+1 for spin s). a) Calculate the partition function of two such particles if they are bosons and also if they are fermions. b ...
... 1. For a single quantum particle of mass m, spectra p2/2m in a volume V the partition fundtion is Z1(m)=gV/3 with h / 2mk BT . The particle has a spin degeneracy g (g=2s+1 for spin s). a) Calculate the partition function of two such particles if they are bosons and also if they are fermions. b ...
Unification of Quantum Statistics ? It`s possible with quaternions to
... likely the old statistics if it's around it seems possible that old statistics can flip one to another changing the minus sign like above. But it' s a really flip in statistics or not? In this moment I not simple to make this statement we must make deepest considerations to solve the question, bu ...
... likely the old statistics if it's around it seems possible that old statistics can flip one to another changing the minus sign like above. But it' s a really flip in statistics or not? In this moment I not simple to make this statement we must make deepest considerations to solve the question, bu ...
Energy of Beta Particles - Ioniserende Stralen Practicum
... 4 Calculate the radius r of the circular path of the β particles in the set-up, and thus their speed v0. r = ………… m v0 = ………… m/s If you calculated the speed correctly, the outcome will be much larger than the speed of light (3.0·108 m/s). So, this outcome cannot be the real speed of the β particles ...
... 4 Calculate the radius r of the circular path of the β particles in the set-up, and thus their speed v0. r = ………… m v0 = ………… m/s If you calculated the speed correctly, the outcome will be much larger than the speed of light (3.0·108 m/s). So, this outcome cannot be the real speed of the β particles ...
Density Triangle Method
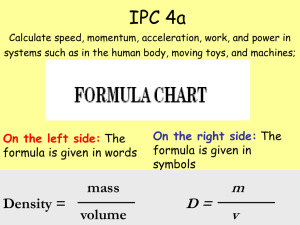
... • The weight lifter used a force of 980 N to raise the barbell 2.04 m over her head in 5.21 seconds. Approximately how much work did she do in raising the barbell? Work = force x distance • Approximately how much power did the weight lifter exert lifting the barbell in 5.21 ...
... • The weight lifter used a force of 980 N to raise the barbell 2.04 m over her head in 5.21 seconds. Approximately how much work did she do in raising the barbell? Work = force x distance • Approximately how much power did the weight lifter exert lifting the barbell in 5.21 ...
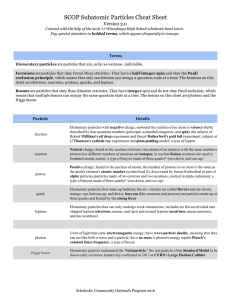
SCOP Subatomic Particles Cheat Sheet
... Fermions are particles that obey FermiDirac statistics. They have a halfinteger spin and obey the Pauli exclusion principle , which means that only one fermion can occupy a quantum state at a time. The fermions on this sheet are ...
... Fermions are particles that obey FermiDirac statistics. They have a halfinteger spin and obey the Pauli exclusion principle , which means that only one fermion can occupy a quantum state at a time. The fermions on this sheet are ...
Complex Numbers Worksheet
... square meters. Gustav weighs 160 pounds and has a BSA of about 2 2m 2 . How tall is he (in cm)? ...
... square meters. Gustav weighs 160 pounds and has a BSA of about 2 2m 2 . How tall is he (in cm)? ...
Worksheet 9 - Impulse
... travelling at 50.0 m/s. Because it is burning fuel, its mass decreases even if it maintains a constant speed. Find its change in momentum if it is still travelling at 50.0 m/s, but now only has 7,000 kg of fuel. ...
... travelling at 50.0 m/s. Because it is burning fuel, its mass decreases even if it maintains a constant speed. Find its change in momentum if it is still travelling at 50.0 m/s, but now only has 7,000 kg of fuel. ...
BASICS OF BOSE-EINSTEIN CONDENSATION THEORY Y. Castin
... Configuration defined by a set of occupation numbers {nα} Example: two spin 1/2 particles of opposite spin: |ψiB ∝ |+i ⊗ |−i + |−i ⊗ |+i |ψiF ∝ |+i ⊗ |−i − |−i ⊗ |+i |+i ⊗ |−i meaningless ...
... Configuration defined by a set of occupation numbers {nα} Example: two spin 1/2 particles of opposite spin: |ψiB ∝ |+i ⊗ |−i + |−i ⊗ |+i |ψiF ∝ |+i ⊗ |−i − |−i ⊗ |+i |+i ⊗ |−i meaningless ...
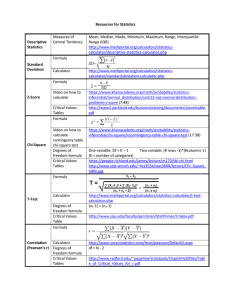
Resources for Statistics Descriptive Statistics Measures of Central
... https://www.khanacademy.org/math/probability/statisticsinferential/chi-square/v/contingency-table-chi-square-test (17:38) One-variable: Df = K – 1 Two variable: (# rows -1)*(#columns-1) (K = number of categories) https://people.richland.edu/james/lecture/m170/tbl-chi.html http://www.stat.wmich.edu/~ ...
... https://www.khanacademy.org/math/probability/statisticsinferential/chi-square/v/contingency-table-chi-square-test (17:38) One-variable: Df = K – 1 Two variable: (# rows -1)*(#columns-1) (K = number of categories) https://people.richland.edu/james/lecture/m170/tbl-chi.html http://www.stat.wmich.edu/~ ...
Empirical Formula/Molecular Formula
... tells actual # of each kind of atom in a molecule of the cmpd. either the same as its experimentally determined empirical formula, or a simple whole‐number multiple of empirical formula ...
... tells actual # of each kind of atom in a molecule of the cmpd. either the same as its experimentally determined empirical formula, or a simple whole‐number multiple of empirical formula ...
Tutorial 1 - NUS Physics Department
... 7. In reactions of the type A B A C1 C2 (in which particle A scatters off particle B, producing C1 , C2 ,) , there is another inertial frame [besides the lab (B at rest) and the CM (PTOT = 0 )] which is sometimes useful. It is called the Breit, or “brick wall,” frame, and it is the syst ...
... 7. In reactions of the type A B A C1 C2 (in which particle A scatters off particle B, producing C1 , C2 ,) , there is another inertial frame [besides the lab (B at rest) and the CM (PTOT = 0 )] which is sometimes useful. It is called the Breit, or “brick wall,” frame, and it is the syst ...
Percent composition
... tells actual # of each kind of atom in a molecule of the cmpd. either the same as its experimentally determined empirical formula, or a simple whole‐number multiple of empirical formula ...
... tells actual # of each kind of atom in a molecule of the cmpd. either the same as its experimentally determined empirical formula, or a simple whole‐number multiple of empirical formula ...
Thesis Presentation Mr. Joshuah T. Heath Department of Physics
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
Document
... 10. The formula I Prt can be used to determine the interest I that is earned on a principal amount of money P, when the money is invested at an annual percentage rate r for t years. a. Solve the formula I Prt for t. ...
... 10. The formula I Prt can be used to determine the interest I that is earned on a principal amount of money P, when the money is invested at an annual percentage rate r for t years. a. Solve the formula I Prt for t. ...
FINAL REVIEW 1st SEMESTER 2014-2015
... aluminum acetate + sodium hydroxide → aluminum hydroxide + sodium acetate ________________________________________________________ ...
... aluminum acetate + sodium hydroxide → aluminum hydroxide + sodium acetate ________________________________________________________ ...
Exercises in Statistical Mechanics
... Reσ(ω) = kB T 0 (c) Write the Diffusion constant D in terms of the velocity-velocity correlation function, assuming that this correlation has a finite range in time. Use Kubo’s formula from (b) in the DC limit of zero frequency to derive the Einstein-Nernst formula for the σ mobility µ = ne = eD/kB ...
... Reσ(ω) = kB T 0 (c) Write the Diffusion constant D in terms of the velocity-velocity correlation function, assuming that this correlation has a finite range in time. Use Kubo’s formula from (b) in the DC limit of zero frequency to derive the Einstein-Nernst formula for the σ mobility µ = ne = eD/kB ...