To find the average number of particles in each state
... Since we know that particles are really “wavicles” and Maxwell Boltzmann statistics is only good for distinguishable particles, what good is it? The Maxwell-Boltzmann distribution assumes that the particles they describe are distinguishable. Two particles can be considered distinguishable if the di ...
... Since we know that particles are really “wavicles” and Maxwell Boltzmann statistics is only good for distinguishable particles, what good is it? The Maxwell-Boltzmann distribution assumes that the particles they describe are distinguishable. Two particles can be considered distinguishable if the di ...
Bose–Einstein condensation NEW PROBLEMS
... physics courses beyond the introductory level. We will publish worked problems that convey the excitement and interest of current developments in physics and that are useful for teaching courses such as Classical Mechanics, Electricity and Magnetism, Statistical Mechanics and Thermodynamics, Modern ...
... physics courses beyond the introductory level. We will publish worked problems that convey the excitement and interest of current developments in physics and that are useful for teaching courses such as Classical Mechanics, Electricity and Magnetism, Statistical Mechanics and Thermodynamics, Modern ...
lecture_11
... Then the number of ways is which the above arrangement can be done is just 1 ! Supposing an arrangement with only one ball occupying each level is desired, then again there only one possible way to obtain it in the indistinguishable balls case. Whereas with distinguishable balls there are N! possibl ...
... Then the number of ways is which the above arrangement can be done is just 1 ! Supposing an arrangement with only one ball occupying each level is desired, then again there only one possible way to obtain it in the indistinguishable balls case. Whereas with distinguishable balls there are N! possibl ...
Two-particle systems
... mechanics, you simply can't say which electron is which as you can not put any labels on them to tell them apart. There are two possible ways to deal with indistinguishable particles, i.e. to construct two-particle wave function that is non committal to which particle is in which state: ...
... mechanics, you simply can't say which electron is which as you can not put any labels on them to tell them apart. There are two possible ways to deal with indistinguishable particles, i.e. to construct two-particle wave function that is non committal to which particle is in which state: ...
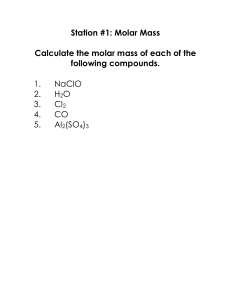
Station #1: Molar Mass
... 2) Find the empirical formula of each compound from its percent composition. a) 65.2% Sc and 34.8% O b) calculate the molecular formula, if the molecular formula mass is 551.68 g. ...
... 2) Find the empirical formula of each compound from its percent composition. a) 65.2% Sc and 34.8% O b) calculate the molecular formula, if the molecular formula mass is 551.68 g. ...
General Chemistry, 5th ed. Whitten, Davis & Peck
... The mass, in atomic mass units, of one formula unit of substance. Numerically equal to the mass, in grams, of one mole of the substance. This number is obtained by adding the atomic weights of the atoms specified in the formula. ...
... The mass, in atomic mass units, of one formula unit of substance. Numerically equal to the mass, in grams, of one mole of the substance. This number is obtained by adding the atomic weights of the atoms specified in the formula. ...
Chapter 10 - WordPress.com
... element in the compound. 2. Find the molar mass of the entire compound. 3. Divide the total molar mass of each element by the molar mass of the compound then multiply by 100 4. Check that all your percentages add up to 100 ...
... element in the compound. 2. Find the molar mass of the entire compound. 3. Divide the total molar mass of each element by the molar mass of the compound then multiply by 100 4. Check that all your percentages add up to 100 ...
How to set up formula I
... What happens to B, if you increase c ? Which of these quantities are (in)directly proportional to B? How to interpret formulas III In a book you find a formula for a physical quantity named M. It says: M= r·g What happens to M, if you increase r ? What happens to M, if you increase g ? Which of the ...
... What happens to B, if you increase c ? Which of these quantities are (in)directly proportional to B? How to interpret formulas III In a book you find a formula for a physical quantity named M. It says: M= r·g What happens to M, if you increase r ? What happens to M, if you increase g ? Which of the ...
Notes - Introduction to Moles: One and Two Step Mole Calculations
... Measuring Matter: There are three ways to measure matter: by counting representative particles (typically molecules or formula units), by mass (in grams), or by volume (in liters for gases). The method used is usually chosen by the ease of each method and the information needed. Once a measurement h ...
... Measuring Matter: There are three ways to measure matter: by counting representative particles (typically molecules or formula units), by mass (in grams), or by volume (in liters for gases). The method used is usually chosen by the ease of each method and the information needed. Once a measurement h ...
Notes - Chemical Quantities
... Empirical Formulas:The empirical formula is the simplest whole number ratio of the atoms of each element in a compound. Note: it is not necessarily the true formula of the compound. For example, the molecular formula for glucose is ________, but its empirical formula is _________. Empirical formulas ...
... Empirical Formulas:The empirical formula is the simplest whole number ratio of the atoms of each element in a compound. Note: it is not necessarily the true formula of the compound. For example, the molecular formula for glucose is ________, but its empirical formula is _________. Empirical formulas ...
Notes - Chemical Quantities
... Empirical Formulas:The empirical formula is the simplest whole number ratio of the atoms of each element in a compound. Note: it is not necessarily the true formula of the compound. For example, the molecular formula for glucose is ________, but its empirical formula is _________. Empirical formulas ...
... Empirical Formulas:The empirical formula is the simplest whole number ratio of the atoms of each element in a compound. Note: it is not necessarily the true formula of the compound. For example, the molecular formula for glucose is ________, but its empirical formula is _________. Empirical formulas ...
Notes - Chemical Quantities
... Empirical Formulas:The empirical formula is the simplest whole number ratio of the atoms of each element in a compound. Note: it is not necessarily the true formula of the compound. For example, the molecular formula for glucose is ________, but its empirical formula is _________. Empirical formulas ...
... Empirical Formulas:The empirical formula is the simplest whole number ratio of the atoms of each element in a compound. Note: it is not necessarily the true formula of the compound. For example, the molecular formula for glucose is ________, but its empirical formula is _________. Empirical formulas ...
Aalborg Universitet The Landauer-Büttiker formula and resonant quantum transport
... In the previous section we have allowed the coupling constant τ (see (3)) to be arbitrarily large. The only assumption was that {−2tL, 2tL } was not in the point spectrum of K. We now look at the small coupling case, τ → 0. In this case we will assume that the sample Hamiltonian H S does not have ei ...
... In the previous section we have allowed the coupling constant τ (see (3)) to be arbitrarily large. The only assumption was that {−2tL, 2tL } was not in the point spectrum of K. We now look at the small coupling case, τ → 0. In this case we will assume that the sample Hamiltonian H S does not have ei ...
Unfair coin
... What is the average Boltzmann occupancy as a function of temperature and chemical potential? Show that if the average Bose and Fermi occupancy is small compared to unity then the Bose and Fermi occupancies reduce to the Boltzmann occupancy. ...
... What is the average Boltzmann occupancy as a function of temperature and chemical potential? Show that if the average Bose and Fermi occupancy is small compared to unity then the Bose and Fermi occupancies reduce to the Boltzmann occupancy. ...
Unit III- Introduction - Varga
... How do different phases of matter look like at the molecular level? ...
... How do different phases of matter look like at the molecular level? ...
Maths Extended - Chapter 8 - Pearson-Global
... A formula is a general rule that shows how quantities (or variables) are related to each other. For example, v 5 u 1 at This is a formula that shows the relationship between an object’s final velocity, v, its initial velocity, u, its acceleration, a, and the time it has been moving, t. ...
... A formula is a general rule that shows how quantities (or variables) are related to each other. For example, v 5 u 1 at This is a formula that shows the relationship between an object’s final velocity, v, its initial velocity, u, its acceleration, a, and the time it has been moving, t. ...
Percentage Composition and Empirical Formulas
... potassium and sulfur do not the same atomic mass the ratio of the masses does not indicate their mole relationships. ...
... potassium and sulfur do not the same atomic mass the ratio of the masses does not indicate their mole relationships. ...
Unit 3 Review (Brinkmann) unit_3_compounds_review1
... Draw the Lewis Symbols of the following molecules. Only single bonds are used. State whether each molecule below is polar or non-polar. ...
... Draw the Lewis Symbols of the following molecules. Only single bonds are used. State whether each molecule below is polar or non-polar. ...
Quantum Grand Canonical Ensemble
... the classical probability distribution, there is no N ! because the combinatorical factors are taken care of in the definition of the quantum state vectors. This holds whether the particles are bosons or fermions. Because ...
... the classical probability distribution, there is no N ! because the combinatorical factors are taken care of in the definition of the quantum state vectors. This holds whether the particles are bosons or fermions. Because ...
How does mathematics Affect science?
... If an atom has 5 protons, 6 neutrons and 5 electrons what would the overall charge be? Think of it this way: Neutrons have no charge so don’t affect the charge. The protons and electrons do however. Turn it into an equation. LET x = charge of atom ...
... If an atom has 5 protons, 6 neutrons and 5 electrons what would the overall charge be? Think of it this way: Neutrons have no charge so don’t affect the charge. The protons and electrons do however. Turn it into an equation. LET x = charge of atom ...
Statistical Physics
... Quantum Statistics Comparison of Distribution Functions The symmetric wave functions describe bosons while the anti-symmetric ones describe fermions. Using these wave functions one can deduce the following: 1. A boson in a quantum state increases the chance of finding other identical bosons in the ...
... Quantum Statistics Comparison of Distribution Functions The symmetric wave functions describe bosons while the anti-symmetric ones describe fermions. Using these wave functions one can deduce the following: 1. A boson in a quantum state increases the chance of finding other identical bosons in the ...
Statistical Mechanics
... Remember, bosons are particles with integral spins. There is no limit on the number of bosons which may occupy any particular state (typically the ground state). Examples are photons in a cavity, phonons, and liquid 4He. Also remember, fermions are particles with half integral spin, with only one pa ...
... Remember, bosons are particles with integral spins. There is no limit on the number of bosons which may occupy any particular state (typically the ground state). Examples are photons in a cavity, phonons, and liquid 4He. Also remember, fermions are particles with half integral spin, with only one pa ...
This handout - Meridian Academy
... form negatively charged ions (anions). This is why water alone does not conduct electricity, but will if salt (NaCl for one) is dissolved in it. Importantly, when these same metal and non-metal particles are combined to form compounds they do not conduct electricity as solids. (How is this similar t ...
... form negatively charged ions (anions). This is why water alone does not conduct electricity, but will if salt (NaCl for one) is dissolved in it. Importantly, when these same metal and non-metal particles are combined to form compounds they do not conduct electricity as solids. (How is this similar t ...