Momentum Do photons carry momentum ? DeBroglie`s Relation
... Shortest wavelengths (Most energetic photons) ...
... Shortest wavelengths (Most energetic photons) ...
AP Physics HW Name: Photon Scattering and X
... The electron encounters a particle with the same mass and opposite charge (a positron) moving with the same speed in the opposite direction. The two particles undergo a head-on collision, which results in the disappearance of both particles and the production of two photons of the same energy. (c) D ...
... The electron encounters a particle with the same mass and opposite charge (a positron) moving with the same speed in the opposite direction. The two particles undergo a head-on collision, which results in the disappearance of both particles and the production of two photons of the same energy. (c) D ...
Chp.23 Outline - Redlands High School
... 8) Explain the cause of spectral lines produced by an excited gas. What does the color of each line depend upon? Why does each element have its own set of spectral lines? 9) Is an electron more wavelike or particle like? Use this to explain why electrons can only exist at certain distances from the ...
... 8) Explain the cause of spectral lines produced by an excited gas. What does the color of each line depend upon? Why does each element have its own set of spectral lines? 9) Is an electron more wavelike or particle like? Use this to explain why electrons can only exist at certain distances from the ...
PHYSICS 113 Assignment #2 SOLUTIONS Chapter 4 1. What
... 12. Why is it dangerous to be exposed to x-rays but not (or at least as much) dangerous to be exposed to radio waves? According to the formula, the energy (E) of a photon (particle of radiation) is proportional to the frequency (<) of the photon. Note that the frequency is inversely proportional to ...
... 12. Why is it dangerous to be exposed to x-rays but not (or at least as much) dangerous to be exposed to radio waves? According to the formula, the energy (E) of a photon (particle of radiation) is proportional to the frequency (<) of the photon. Note that the frequency is inversely proportional to ...
Photon gas as a classical medium
... takes the simple meaning of the Compton wavelength of a photon in a plasma In the relativistic theory a coordinate uncertainty in a frame of reference in which the particle is moving with energy ...
... takes the simple meaning of the Compton wavelength of a photon in a plasma In the relativistic theory a coordinate uncertainty in a frame of reference in which the particle is moving with energy ...
A progressive electromagnetic wave is a self
... (photons) moving at the speed of light. Each photon carries very little energy. However, even an ordinary torch beam is a torrent of ~1017 photons.s-1. When we “see” light what we observe by eye or on film is the average energy per unit area per time arriving at some surface. ...
... (photons) moving at the speed of light. Each photon carries very little energy. However, even an ordinary torch beam is a torrent of ~1017 photons.s-1. When we “see” light what we observe by eye or on film is the average energy per unit area per time arriving at some surface. ...
Lecture 11
... (photons) moving at the speed of light. Each photon carries very little energy. However, even an ordinary torch beam is a torrent of ~1017 photons.s-1. When we “see” light what we observe by eye or on film is the average energy per unit area per time arriving at some surface. ...
... (photons) moving at the speed of light. Each photon carries very little energy. However, even an ordinary torch beam is a torrent of ~1017 photons.s-1. When we “see” light what we observe by eye or on film is the average energy per unit area per time arriving at some surface. ...
photon particle - wave duality
... 1. a. Read sections 2.1 and 2.2 of the text. What is the ultraviolet catastrophe of the Rayleigh-Jeans law (equation 2.5)? b. Read section 2.3 paying particular attention to assumption (1) and (2) on page 47. What does the Maxwell - Boltzmann distribution function (equation 2.6) tell you about the n ...
... 1. a. Read sections 2.1 and 2.2 of the text. What is the ultraviolet catastrophe of the Rayleigh-Jeans law (equation 2.5)? b. Read section 2.3 paying particular attention to assumption (1) and (2) on page 47. What does the Maxwell - Boltzmann distribution function (equation 2.6) tell you about the n ...
PPT | 345.5 KB - Joint Quantum Institute
... Physicists supported by the PFC at the Joint Quantum Institute have developed a new source of “entangled” photons – fundamental units of light whose properties are so intertwined that if the condition of one is measured, the condition of the other is instantaneously known, even if the photons are th ...
... Physicists supported by the PFC at the Joint Quantum Institute have developed a new source of “entangled” photons – fundamental units of light whose properties are so intertwined that if the condition of one is measured, the condition of the other is instantaneously known, even if the photons are th ...
1 EM Waves Intro
... All travel at the same speed but, EM waves are differentiated by wavelength, frequency, energy ...
... All travel at the same speed but, EM waves are differentiated by wavelength, frequency, energy ...
Quantum Theory Chapter 27
... max KE of the electrons at the cathode is equal to at the anode • KE = -q Vo • Vo is the magnitude of the stopping potential in J/C and q is the charge of an electron in C. • Since a Joule is so large for atomic systems we ...
... max KE of the electrons at the cathode is equal to at the anode • KE = -q Vo • Vo is the magnitude of the stopping potential in J/C and q is the charge of an electron in C. • Since a Joule is so large for atomic systems we ...
Class 11 I : The speed of light
... magnetism (Maxwell’s equations). Electric and magnetic fields are just facets of a unified electromagnetic field Immediate prediction of Maxwell’s equation… waves of electromagnetic energy can travel through vacuum with a speed of 3.0x108m/s. He realized that he had just “discovered” light These wav ...
... magnetism (Maxwell’s equations). Electric and magnetic fields are just facets of a unified electromagnetic field Immediate prediction of Maxwell’s equation… waves of electromagnetic energy can travel through vacuum with a speed of 3.0x108m/s. He realized that he had just “discovered” light These wav ...
Bohr Model of Hydrogen
... Bohr’s Model • 1. All forms of energy are quantized (only fixed, discrete amounts or quanta). • 2. Electron can only occupy certain specific CIRCULAR orbits of fixed radius and none other. • 3. Electron can jump from one orbit to a higher one by absorbing the EXACT quantum of energy in form of a ph ...
... Bohr’s Model • 1. All forms of energy are quantized (only fixed, discrete amounts or quanta). • 2. Electron can only occupy certain specific CIRCULAR orbits of fixed radius and none other. • 3. Electron can jump from one orbit to a higher one by absorbing the EXACT quantum of energy in form of a ph ...
Lasers - eochemistry
... Then it is inserted or combined with the excited atom This combination results in the production of more photons ...
... Then it is inserted or combined with the excited atom This combination results in the production of more photons ...
QUIZ 4 ... Formulas and constants Mass of electron = 9.1. 10
... Coulomb’s constant k 1 / (4 0 ) 8.99.109 N.m2 / kg2 Velocity of light c = 3.108 m/s Bohr’s quantization for Angular momentum mvr nh Bohr radius a0 =0.529. 10-10 m = (h-bar) 2/meke2 1 Rydberg (Energy required to ionize hydrogen atom) =13.6 eV Rydberg Constant R = 1.097. 107 m-1 Energy of phot ...
... Coulomb’s constant k 1 / (4 0 ) 8.99.109 N.m2 / kg2 Velocity of light c = 3.108 m/s Bohr’s quantization for Angular momentum mvr nh Bohr radius a0 =0.529. 10-10 m = (h-bar) 2/meke2 1 Rydberg (Energy required to ionize hydrogen atom) =13.6 eV Rydberg Constant R = 1.097. 107 m-1 Energy of phot ...
Synopsis
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...
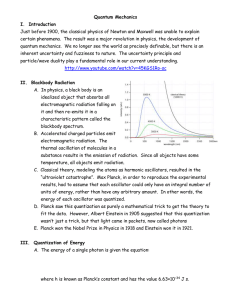
Quantum Mechanics I. Introduction Just before 1900, the classical
... A. In physics, a black body is an idealized object that absorbs all electromagnetic radiation falling on it and then re-emits it in a characteristic pattern called the blackbody spectrum. B. Accelerated charged particles emit electromagnetic radiation. The thermal oscillation of molecules in a subst ...
... A. In physics, a black body is an idealized object that absorbs all electromagnetic radiation falling on it and then re-emits it in a characteristic pattern called the blackbody spectrum. B. Accelerated charged particles emit electromagnetic radiation. The thermal oscillation of molecules in a subst ...
Phys202_Exam3_2006.doc
... 26. Why was it so revolutionary? a. it predicted the quantized state of the photon b. it explained the photoelectric effect c. ~ it made matter into a wave whereas it had always been thought of as a particle d. it showed that there was no negative mass state 27. What explicitly did Heisenberg propos ...
... 26. Why was it so revolutionary? a. it predicted the quantized state of the photon b. it explained the photoelectric effect c. ~ it made matter into a wave whereas it had always been thought of as a particle d. it showed that there was no negative mass state 27. What explicitly did Heisenberg propos ...
6.1.1
... The Birth of Classical Physics • The ancient Greeks were certainly not the first to wonder about and investigate nature, but they were the first to leave written records of their ideas. • They recorded ideas regarding a vast number of subjects from Astronomy to Zoology. • They conceptualized the bu ...
... The Birth of Classical Physics • The ancient Greeks were certainly not the first to wonder about and investigate nature, but they were the first to leave written records of their ideas. • They recorded ideas regarding a vast number of subjects from Astronomy to Zoology. • They conceptualized the bu ...
A system consist of two particles,each of which has two possible
... wave vector k is composed of photons of energy and momentum p= k .Photons are massless bosons.Since photons are continuously emitted and absorbed by the walls , the number of photons in the box continuously change. Since photons are massless , the chemical potential is zero. (a)Find the average ...
... wave vector k is composed of photons of energy and momentum p= k .Photons are massless bosons.Since photons are continuously emitted and absorbed by the walls , the number of photons in the box continuously change. Since photons are massless , the chemical potential is zero. (a)Find the average ...
Quantum Nature of Light
... Spectrum of the photons emitted by a LED Driver and detected by a Silicon ...
... Spectrum of the photons emitted by a LED Driver and detected by a Silicon ...
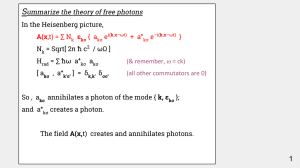
S
... A(x,t) = ∑ Nk εkσ { akσ ei(k.x–ωt) + a+kσ e–i(k.x–ωt) } Nk = Sqrt[ 2π ħ c2 / ωΩ ] Hrad = ∑ ħω a+kσ akσ ...
... A(x,t) = ∑ Nk εkσ { akσ ei(k.x–ωt) + a+kσ e–i(k.x–ωt) } Nk = Sqrt[ 2π ħ c2 / ωΩ ] Hrad = ∑ ħω a+kσ akσ ...
Photon Fishing
... Department of Physics, University of Queensland, QLD 4072, Brisbane, Australia. There is considerable interest in experimentally implementing quantum information (QI) protocols and quantum computation. It has been proposed that a quantum computer can be built solely with linear optics, single photon ...
... Department of Physics, University of Queensland, QLD 4072, Brisbane, Australia. There is considerable interest in experimentally implementing quantum information (QI) protocols and quantum computation. It has been proposed that a quantum computer can be built solely with linear optics, single photon ...
Photon
A photon is an elementary particle, the quantum of light and all other forms of electromagnetic radiation. It is the force carrier for the electromagnetic force, even when static via virtual photons. The effects of this force are easily observable at the microscopic and at the macroscopic level, because the photon has zero rest mass; this allows long distance interactions. Like all elementary particles, photons are currently best explained by quantum mechanics and exhibit wave–particle duality, exhibiting properties of waves and of particles. For example, a single photon may be refracted by a lens or exhibit wave interference with itself, but also act as a particle giving a definite result when its position is measured. Waves and quanta, being two observable aspects of a single phenomenon cannot have their true nature described in terms of any mechanical model. A representation of this dual property of light, which assumes certain points on the wave front to be the seat of the energy is also impossible. Thus, the quanta in a light wave cannot be spatially localized. Some defined physical parameters of a photon are listed. The modern photon concept was developed gradually by Albert Einstein in the first years of the 20th century to explain experimental observations that did not fit the classical wave model of light. In particular, the photon model accounted for the frequency dependence of light's energy, and explained the ability of matter and radiation to be in thermal equilibrium. It also accounted for anomalous observations, including the properties of black-body radiation, that other physicists, most notably Max Planck, had sought to explain using semiclassical models, in which light is still described by Maxwell's equations, but the material objects that emit and absorb light do so in amounts of energy that are quantized (i.e., they change energy only by certain particular discrete amounts and cannot change energy in any arbitrary way). Although these semiclassical models contributed to the development of quantum mechanics, many further experiments starting with Compton scattering of single photons by electrons, first observed in 1923, validated Einstein's hypothesis that light itself is quantized. In 1926 the optical physicist Frithiof Wolfers and the chemist Gilbert N. Lewis coined the name photon for these particles, and after 1927, when Arthur H. Compton won the Nobel Prize for his scattering studies, most scientists accepted the validity that quanta of light have an independent existence, and the term photon for light quanta was accepted.In the Standard Model of particle physics, photons and other elementary particles are described as a necessary consequence of physical laws having a certain symmetry at every point in spacetime. The intrinsic properties of particles, such as charge, mass and spin, are determined by the properties of this gauge symmetry.The photon concept has led to momentous advances in experimental and theoretical physics, such as lasers, Bose–Einstein condensation, quantum field theory, and the probabilistic interpretation of quantum mechanics. It has been applied to photochemistry, high-resolution microscopy, and measurements of molecular distances. Recently, photons have been studied as elements of quantum computers and for applications in optical imaging and optical communication such as quantum cryptography.