Heavy-quark energy loss in finite extend SYM plasma
... - the part of the string below Qs is not causally connected with the part of the string above: Qs corresponds to a horizon in the rest frame of the string - when computing the stress-tensor on the boundary: the trailing string is a source of metric perturbations in the bulk which give ...
... - the part of the string below Qs is not causally connected with the part of the string above: Qs corresponds to a horizon in the rest frame of the string - when computing the stress-tensor on the boundary: the trailing string is a source of metric perturbations in the bulk which give ...
Kinetic Energy - SJSU Engineering
... • We build machines to ‘manage’ energy conversion. • While energy cannot be destroyed, once it is transformed into a certain form (heat, often the case), it is basically difficult to use (some say “lost” but we cannot actually lose energy). based on notes of P. Hsu 2007 ...
... • We build machines to ‘manage’ energy conversion. • While energy cannot be destroyed, once it is transformed into a certain form (heat, often the case), it is basically difficult to use (some say “lost” but we cannot actually lose energy). based on notes of P. Hsu 2007 ...
Energy - TeacherWeb
... ___________ mechanical 2. The energy that deals with motion is ___________ and ____________. K.E. electromagnetic 3. The energy that includes light is ______________. position 4. Potential energy depends on the ____________ of an object and its ____________ chemical composition. 5. Kinetic energy de ...
... ___________ mechanical 2. The energy that deals with motion is ___________ and ____________. K.E. electromagnetic 3. The energy that includes light is ______________. position 4. Potential energy depends on the ____________ of an object and its ____________ chemical composition. 5. Kinetic energy de ...
1st Year Thermodynamic Lectures Dr Mark R. Wormald
... q - heat absorbed by the system from the surroundings w - work done on the system by the surroundings ∆Usystem = q + w Distinction between work and heat :Work is the energy associated with orderly movements of bodies, for example pushing back boundaries (volume change) or moving electrons along a wi ...
... q - heat absorbed by the system from the surroundings w - work done on the system by the surroundings ∆Usystem = q + w Distinction between work and heat :Work is the energy associated with orderly movements of bodies, for example pushing back boundaries (volume change) or moving electrons along a wi ...
Energy Lesson
... chemical to thermal and light sound to electrical electrical to sound and thermal electrical to sound and light and thermal chemical to kinetic and sound and thermal ...
... chemical to thermal and light sound to electrical electrical to sound and thermal electrical to sound and light and thermal chemical to kinetic and sound and thermal ...
Pearson Science 8 Student Book, Unit 5.1 - Energy
... running blades could give him an unfair advantage over able-bodied athletes. Pistorius was able to enter the qualifying races for the 2008 Beijing Olympics after a court decided that there was not enough evidence to prove that his blades gave him any advantage. ...
... running blades could give him an unfair advantage over able-bodied athletes. Pistorius was able to enter the qualifying races for the 2008 Beijing Olympics after a court decided that there was not enough evidence to prove that his blades gave him any advantage. ...
Topic 6_2_Ext D__Electric potential energy and
... FYI: Perhaps you recall that only CONSERVATIVE forces can have Topic The ...
... FYI: Perhaps you recall that only CONSERVATIVE forces can have Topic The ...
The Motionless Electromagnetic Generator: How It
... sum to zero vectorially. Hence no external work is done, but internal work is done on the system to produce and continuously maintain this stress with zero translation. Work is not the change of magnitude of energy in a single form! It is the change of form of energy, from one form to another. Thus ...
... sum to zero vectorially. Hence no external work is done, but internal work is done on the system to produce and continuously maintain this stress with zero translation. Work is not the change of magnitude of energy in a single form! It is the change of form of energy, from one form to another. Thus ...
05Thermal_PhysicsALT
... The rest of the universe is called the “environment” or the “surroundings”. • Isolated system: No matter or energy is exchanged with the environment. (ex: thermos) • Closed system (or “control mass”): no matter is exchanged with the environment. (ex: gas in a cylinder with a piston.) • Open system ( ...
... The rest of the universe is called the “environment” or the “surroundings”. • Isolated system: No matter or energy is exchanged with the environment. (ex: thermos) • Closed system (or “control mass”): no matter is exchanged with the environment. (ex: gas in a cylinder with a piston.) • Open system ( ...
LP 2.1: Introduction to Forms of Energy
... gravitational energy is stored. When someone rides a bicycle down a steep hill and picks up speed, the gravitational energy is being converted to motion energy. Hydropower is another example of gravitational energy, where the dam "piles" up water from a river into a reservoir. ...
... gravitational energy is stored. When someone rides a bicycle down a steep hill and picks up speed, the gravitational energy is being converted to motion energy. Hydropower is another example of gravitational energy, where the dam "piles" up water from a river into a reservoir. ...
CH17 notes
... Potential Energy is an energy associated with the interaction of a pair of particles within a system. ...
... Potential Energy is an energy associated with the interaction of a pair of particles within a system. ...
Chapter 10
... • The Law of Conservation of Energy is also known as The First Law of Thermodynamics. It can be stated as “the energy of the universe is constant.” • Internal Energy (E) = kinetic energy + potential energy • ΔE = q + w = change in internal energy q = heat absorbed by the system w = work done on the ...
... • The Law of Conservation of Energy is also known as The First Law of Thermodynamics. It can be stated as “the energy of the universe is constant.” • Internal Energy (E) = kinetic energy + potential energy • ΔE = q + w = change in internal energy q = heat absorbed by the system w = work done on the ...
JOURNAL DE PHYSIQUE Colloque C2, supplement au n03, Tome 47,
... where KaB = 2.25 exp(-0.13 ~-l IRa - RBI). Because the atomic orbitals lP~ are nonorthogonal. we cannot account for the atomic polarization effects in (3) but only include field effects with the energy shift in (2). In our calculation we keep the geometry of the Fe 4 cluster fixed and vary the nitro ...
... where KaB = 2.25 exp(-0.13 ~-l IRa - RBI). Because the atomic orbitals lP~ are nonorthogonal. we cannot account for the atomic polarization effects in (3) but only include field effects with the energy shift in (2). In our calculation we keep the geometry of the Fe 4 cluster fixed and vary the nitro ...
Lecture 2
... A rising piston, a rotating shaft, and an electric wire crossing the system boundaries are all associated with work interactions Formal sign convention: Heat transfer to a system and work done by a system are positive; heat transfer from a system and work done on a system are negative. Alterna ...
... A rising piston, a rotating shaft, and an electric wire crossing the system boundaries are all associated with work interactions Formal sign convention: Heat transfer to a system and work done by a system are positive; heat transfer from a system and work done on a system are negative. Alterna ...
Intro_1
... – Thermal – temperature same at all points of system – Mechanical – pressure same at all points of system at all time – Phase – mass of each phase about the same – Chemical – chemical reaction stop ...
... – Thermal – temperature same at all points of system – Mechanical – pressure same at all points of system at all time – Phase – mass of each phase about the same – Chemical – chemical reaction stop ...
Chapter #10
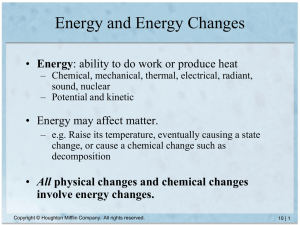
... Although chemistry is the study of matter, matter is effected by energy. – It can cause physical and/or chemical changes in matter. ...
... Although chemistry is the study of matter, matter is effected by energy. – It can cause physical and/or chemical changes in matter. ...
Questions - TTU Physics
... length ℓ. It oscillates in a plane with no friction. The total mechanical energy is E = [(L2)/(2mℓ2)] + mgℓ(1 – cosθ). L is the angular momentum about the suspension point. θ is the oscillation angle. θ = 0 is where the wire is vertical. See figure In what follows, make the small θ approximation ...
... length ℓ. It oscillates in a plane with no friction. The total mechanical energy is E = [(L2)/(2mℓ2)] + mgℓ(1 – cosθ). L is the angular momentum about the suspension point. θ is the oscillation angle. θ = 0 is where the wire is vertical. See figure In what follows, make the small θ approximation ...
Conservation of energy

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. Energy can be neither created nor be destroyed, but it transforms from one form to another, for instance chemical energy can be converted to kinetic energy in the explosion of a stick of dynamite.A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind cannot exist. That is to say, no system without an external energy supply can deliver an unlimited amount of energy to its surroundings.