Chapter 15 Power Point Notes
... unit for mass is kilograms. The unit for height is meters. Acceleration due to gravity, g, has a value in SI units of 9.8 m/s2 on Earth. The unit for gravitational potential energy ...
... unit for mass is kilograms. The unit for height is meters. Acceleration due to gravity, g, has a value in SI units of 9.8 m/s2 on Earth. The unit for gravitational potential energy ...
Work Done - akamdiplomaphysics
... a heat reservoir, and is fitted with a light, frictionless, movable piston If an amount of heat Q is added to the system which is at point A of an isotherm, then the system will move to another point on the graph, B. ...
... a heat reservoir, and is fitted with a light, frictionless, movable piston If an amount of heat Q is added to the system which is at point A of an isotherm, then the system will move to another point on the graph, B. ...
Thermodynamics
... Q 0W DU That leaves First Law as If the gas expands, the internal energy decreases, and so does the temperature ...
... Q 0W DU That leaves First Law as If the gas expands, the internal energy decreases, and so does the temperature ...
Energy Transfers
... (a) What is energy? (b) State the unit of energy. (see page 127) Give examples of the following energy changes: (a) electrical to light; (b) kinetic to sound; (c) nuclear to light; (d) chemical to gravitational potential; (e) elastic potential to thermal. (see pages 128 and 129) State the law of con ...
... (a) What is energy? (b) State the unit of energy. (see page 127) Give examples of the following energy changes: (a) electrical to light; (b) kinetic to sound; (c) nuclear to light; (d) chemical to gravitational potential; (e) elastic potential to thermal. (see pages 128 and 129) State the law of con ...
Charge Carriers in Semiconductors.
... higher anti-bonding state of that bond. In the picture of delocalized states, for example in one dimension - that is in a nanowire, for every energy there is a state with electrons flowing in one direction and one state for the electrons flowing in the other. ...
... higher anti-bonding state of that bond. In the picture of delocalized states, for example in one dimension - that is in a nanowire, for every energy there is a state with electrons flowing in one direction and one state for the electrons flowing in the other. ...
First law of thermodynamics
... 1st Law = Conservation of Energy The first law of thermodynamics is simply an expression of the conservation of energy principle. The principle of the conservation of energy states that energy can neither be created nor destroyed. But it can change from one type of energy to another (for example ki ...
... 1st Law = Conservation of Energy The first law of thermodynamics is simply an expression of the conservation of energy principle. The principle of the conservation of energy states that energy can neither be created nor destroyed. But it can change from one type of energy to another (for example ki ...
Lecture 2
... • Allowed electron energy levels in an atom give rise to bands of allowed electron energy levels in a crystal. – The valence band is the highest nearly-filled band. – The conduction band is the lowest nearly-empty band. ...
... • Allowed electron energy levels in an atom give rise to bands of allowed electron energy levels in a crystal. – The valence band is the highest nearly-filled band. – The conduction band is the lowest nearly-empty band. ...
Discussion paper on calorific values
... According to the Energy Statistics Manual, “energy content of solid and liquid fossil fuels, and of renewables and wastes is expressed in net calorific value (NCV). Energy content of natural gas and manufactured gases is expressed in gross calorific value (GCV)”. For gases, these values are converte ...
... According to the Energy Statistics Manual, “energy content of solid and liquid fossil fuels, and of renewables and wastes is expressed in net calorific value (NCV). Energy content of natural gas and manufactured gases is expressed in gross calorific value (GCV)”. For gases, these values are converte ...
Practice Midterm Test 1
... Problem: A proton is fired a proton with a speed of 200 000 m/s from the midpoint of the capacitor toward the positive plate. (a) show that this is insufficient field to reach the positive plat. (b) What is the proton’s speed as it collides with the negative plate? Energy is conserved. The proton’s ...
... Problem: A proton is fired a proton with a speed of 200 000 m/s from the midpoint of the capacitor toward the positive plate. (a) show that this is insufficient field to reach the positive plat. (b) What is the proton’s speed as it collides with the negative plate? Energy is conserved. The proton’s ...
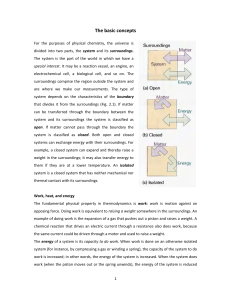
The basic concepts For the purposes of physical chemistry, the
... a process in which energy is acquired from its surroundings as heat. An example of an endothermic process is the vaporization of water. To avoid a lot of awkward circumlocution, we say that in an exothermic process energy is transferred 'as heat' to the surroundings and in an endothermic process ene ...
... a process in which energy is acquired from its surroundings as heat. An example of an endothermic process is the vaporization of water. To avoid a lot of awkward circumlocution, we say that in an exothermic process energy is transferred 'as heat' to the surroundings and in an endothermic process ene ...
Energy:
... Kinetic-Potential Energy Conversion Roller coasters work because of the energy that is built into the system. Initially, the cars are pulled mechanically up the tallest hill, giving them a great deal of potential energy. From that point, the conversion between potential and kinetic energy powers th ...
... Kinetic-Potential Energy Conversion Roller coasters work because of the energy that is built into the system. Initially, the cars are pulled mechanically up the tallest hill, giving them a great deal of potential energy. From that point, the conversion between potential and kinetic energy powers th ...
Conservation of energy

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. Energy can be neither created nor be destroyed, but it transforms from one form to another, for instance chemical energy can be converted to kinetic energy in the explosion of a stick of dynamite.A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind cannot exist. That is to say, no system without an external energy supply can deliver an unlimited amount of energy to its surroundings.