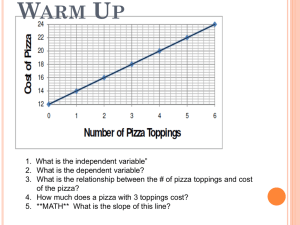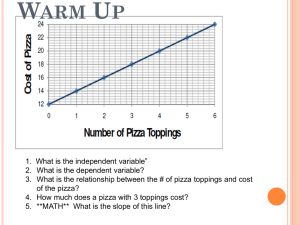
Review Unit 5 Properties of Energy
... chemical energy from the food you ate into mechanical energy. Although energy constantly changes from one form to another, it is never created or destroyed. This is known as the laws of conservation of energy. The law of conservation of energy cannot be broken. When energy changes form or causes cha ...
... chemical energy from the food you ate into mechanical energy. Although energy constantly changes from one form to another, it is never created or destroyed. This is known as the laws of conservation of energy. The law of conservation of energy cannot be broken. When energy changes form or causes cha ...
Energy - Denton ISD
... If an object or organism does work, the object or organism is using energy. ...
... If an object or organism does work, the object or organism is using energy. ...
Notes/All Physics IB/Introductory Items/vocabulary list ib2
... 21. Kinetic Energy (EK) – product of ½ times the mass of an object times the square of an object’s speed 22. Change in Gravitational Potential Energy (ΔEp) – product of an object’s mass times the gravitational field strength times the change in height 23. *Principle of Conservation of Energy – The t ...
... 21. Kinetic Energy (EK) – product of ½ times the mass of an object times the square of an object’s speed 22. Change in Gravitational Potential Energy (ΔEp) – product of an object’s mass times the gravitational field strength times the change in height 23. *Principle of Conservation of Energy – The t ...
Level C - Back to Home Page
... When energy gets changed or transferred from one form to another it is called an energy transfer. We show these energy transfers by using arrows in an energy transfer diagram. Example 1 - A bonfire changes energy which has been stored in the wood into heat and light and even some sound. Energy trans ...
... When energy gets changed or transferred from one form to another it is called an energy transfer. We show these energy transfers by using arrows in an energy transfer diagram. Example 1 - A bonfire changes energy which has been stored in the wood into heat and light and even some sound. Energy trans ...
H - Bruder Chemistry
... made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting over a distance. Heat is energy transferred between objects because of temperature difference. ...
... made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting over a distance. Heat is energy transferred between objects because of temperature difference. ...
Theory of lithographically-induced self-assembly Z. Suo and J. Liang
... systems.7 The instability, however, does not set a stable, uniform island size. Focus on the process in the columnar configuration, in which the two phases can change configuration by lateral flow. When the lateral feature size increases, the total interface energy reduces, but the electrostatic ene ...
... systems.7 The instability, however, does not set a stable, uniform island size. Focus on the process in the columnar configuration, in which the two phases can change configuration by lateral flow. When the lateral feature size increases, the total interface energy reduces, but the electrostatic ene ...
Dynamic system modeling for control and diagnosis
... Mass (m) / amount of substance (n), volume (V), pressure (p), temperature (T) describes the gas state. ...
... Mass (m) / amount of substance (n), volume (V), pressure (p), temperature (T) describes the gas state. ...
The First Law of Thermodynamics
... Many detailed experiments have veri ed that ∆U = Q − W , where ∆U is the change in total kinetic and potential energy of all atoms and molecules in a system. It has also been determined experimentally that the internal energy U of a system depends only on the state of the system and not how it reach ...
... Many detailed experiments have veri ed that ∆U = Q − W , where ∆U is the change in total kinetic and potential energy of all atoms and molecules in a system. It has also been determined experimentally that the internal energy U of a system depends only on the state of the system and not how it reach ...
Energy - Charles W. Davidson College of Engineering
... • We build machines to ‘manage’ energy conversion. • While energy cannot be destroyed, once it is transformed into a certain form (heat, often the case), it is basically difficult to use (some say “lost” but we cannot actually lose energy). based on notes of P. Hsu 2007 ...
... • We build machines to ‘manage’ energy conversion. • While energy cannot be destroyed, once it is transformed into a certain form (heat, often the case), it is basically difficult to use (some say “lost” but we cannot actually lose energy). based on notes of P. Hsu 2007 ...
File
... How many joules of energy does the alternator produce each second? 1 C is given 12J of energy therefor 50 C x 12 J = 600J ...
... How many joules of energy does the alternator produce each second? 1 C is given 12J of energy therefor 50 C x 12 J = 600J ...
Thermodynamics
... boundaries, denoted by U, or sometimes E, is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with the vibrational and electric energy of atoms within molecules or crystals. It includes the energy in all the c ...
... boundaries, denoted by U, or sometimes E, is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with the vibrational and electric energy of atoms within molecules or crystals. It includes the energy in all the c ...
Lecture 2: Energy Balance - San Jose State University
... What is the source of global energy? What is the difference between icesheet and ocean in terms of their reflections on ...
... What is the source of global energy? What is the difference between icesheet and ocean in terms of their reflections on ...
- Mehran Mamonai`s Website
... The principle of work and kinetic energy (also known as the work-energy theorem) states that the work done by the sum of all forces acting on a particle equals the change in the kinetic energy of the particle. Work can change the potential energy of a mechanical device, the heat energy in a ther ...
... The principle of work and kinetic energy (also known as the work-energy theorem) states that the work done by the sum of all forces acting on a particle equals the change in the kinetic energy of the particle. Work can change the potential energy of a mechanical device, the heat energy in a ther ...
Chapter 15: Energy
... Energy and Mass Einstein’s E=mc2, says that energy and mass are equivalent and can be converted into each other. In other words, energy is released as matter is destroyed, and matter can be created from energy. The law of conservation of energy has been modified to say that mass and energy toge ...
... Energy and Mass Einstein’s E=mc2, says that energy and mass are equivalent and can be converted into each other. In other words, energy is released as matter is destroyed, and matter can be created from energy. The law of conservation of energy has been modified to say that mass and energy toge ...
Conservation of energy

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. Energy can be neither created nor be destroyed, but it transforms from one form to another, for instance chemical energy can be converted to kinetic energy in the explosion of a stick of dynamite.A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind cannot exist. That is to say, no system without an external energy supply can deliver an unlimited amount of energy to its surroundings.