7.1 Describing Reactions
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
Slide 1
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
7.1 Describing Reactions
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
7.1 Describing Reactions
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
View PDF
... An electrically neutral atom of mercury (atomic number 80) has a. 80 neutrons and 80 electrons. c. 80 protons and 80 neutrons. b. ...
... An electrically neutral atom of mercury (atomic number 80) has a. 80 neutrons and 80 electrons. c. 80 protons and 80 neutrons. b. ...
History of the Atom
... –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. ...
... –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. ...
CHAPTER 21 NONMETALLIC ELEMENTS AND THEIR COMPOUNDS
... the idea of excessive crowding of the six chlorine, bromine, or iodine atoms around the sulfur. Others suggest that sulfur in the +6 oxidation state would oxidize chlorine, bromine, or iodine in the −1 oxidation state to the free elements. In any case, none of these substances has been made as of th ...
... the idea of excessive crowding of the six chlorine, bromine, or iodine atoms around the sulfur. Others suggest that sulfur in the +6 oxidation state would oxidize chlorine, bromine, or iodine in the −1 oxidation state to the free elements. In any case, none of these substances has been made as of th ...
reactions taking place within cells
... - Organic compounds thermodynamically unstable in the presence of oxygen CH4(l) + 2O2(g) CO2(g) + 2H2O(g) (-)H But activation energies of the reactions with oxygen are high so organic compounds are kinetically stable at temperatures on earth If alkane and oxygen mixed first explosion will result ...
... - Organic compounds thermodynamically unstable in the presence of oxygen CH4(l) + 2O2(g) CO2(g) + 2H2O(g) (-)H But activation energies of the reactions with oxygen are high so organic compounds are kinetically stable at temperatures on earth If alkane and oxygen mixed first explosion will result ...
Mock Examination (2016/2017) CHEMISTRY PAPER 1 SECTION B
... Given the standard enthalpy change of combustion of 2-methylpropan-2-ol is 2643.8 kJ mol1, explain which of the compounds, butan-1-ol or 2-methylpropan-2-ol, is more energetically stable based on the answer in (b). Both are structural isomeric to each other, gives the same number of mole and same ...
... Given the standard enthalpy change of combustion of 2-methylpropan-2-ol is 2643.8 kJ mol1, explain which of the compounds, butan-1-ol or 2-methylpropan-2-ol, is more energetically stable based on the answer in (b). Both are structural isomeric to each other, gives the same number of mole and same ...
5.1 questions - DrBravoChemistry
... Calculate the standard enthalpy change and the standard entropy change for this reaction. Standard enthalpy change ........................................................................... ...
... Calculate the standard enthalpy change and the standard entropy change for this reaction. Standard enthalpy change ........................................................................... ...
SUGGESTED TIMELINE: 4 Weeks - Hazlet Township Public Schools
... atoms of an element and the number of an atom’s subatomic particles can be expressed and measured. It also covers the work of Mendeleev and other chemists in developing the periodic table and explains how the periodic law is used to predict elements’ physical and chemical properties. The relationshi ...
... atoms of an element and the number of an atom’s subatomic particles can be expressed and measured. It also covers the work of Mendeleev and other chemists in developing the periodic table and explains how the periodic law is used to predict elements’ physical and chemical properties. The relationshi ...
P2 11 Nuclear Fission and Fusion
... Scientists cannot give the exact number of years a star will be in the ‘main sequence’ period. Suggest why. ...
... Scientists cannot give the exact number of years a star will be in the ‘main sequence’ period. Suggest why. ...
23. Oxidation and Reduction
... involve polyatomic ions like PO43- or NO 31-. Before we can attempt to understand redox equations that include such ions, we must know how to determine the oxidation number of each atom in a polyatomic ion. This is not new to you. You worked with this concept back in Chapter 14. For example, what is ...
... involve polyatomic ions like PO43- or NO 31-. Before we can attempt to understand redox equations that include such ions, we must know how to determine the oxidation number of each atom in a polyatomic ion. This is not new to you. You worked with this concept back in Chapter 14. For example, what is ...
AP Chemistry Summer Assignment
... haven’t covered yet. Having the following skills will be essential to your success in AP Chemistry and I will expect that you already have a firm grasp on these topics as we start the year. The following assignment is to be completed over the summer and brought in COMPLETED on the first day of class ...
... haven’t covered yet. Having the following skills will be essential to your success in AP Chemistry and I will expect that you already have a firm grasp on these topics as we start the year. The following assignment is to be completed over the summer and brought in COMPLETED on the first day of class ...
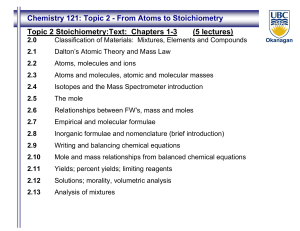
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... mass number and the atomic number, or (A -Z). o For example, the mass number of fluorine is 19 and the atomic number is 9 (indicating 9 protons in the nucleus). Thus the number of neutrons in an atom of fluorine is 19 -9 = 10. The atomic number, number of neutrons, and mass number all must be positi ...
... mass number and the atomic number, or (A -Z). o For example, the mass number of fluorine is 19 and the atomic number is 9 (indicating 9 protons in the nucleus). Thus the number of neutrons in an atom of fluorine is 19 -9 = 10. The atomic number, number of neutrons, and mass number all must be positi ...
Homework Chapter 6
... 23. During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation: 2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g) Calculate the standard enthalpy change for the above reaction given: 3S(s) + 2H2O(g) 2H2S(g) + SO2(g) H° = 146.9 kJ/mol S(s) + O2 ...
... 23. During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation: 2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g) Calculate the standard enthalpy change for the above reaction given: 3S(s) + 2H2O(g) 2H2S(g) + SO2(g) H° = 146.9 kJ/mol S(s) + O2 ...
Chapter 4
... Symbols used in equations (aq) after the formula - dissolved in water, an aqueous solution. used after a product indicates a gas (same as (g)) (products only) used after a product indicates a solid (same as (s)) (products only) ...
... Symbols used in equations (aq) after the formula - dissolved in water, an aqueous solution. used after a product indicates a gas (same as (g)) (products only) used after a product indicates a solid (same as (s)) (products only) ...
Section 4.8: Acid-Base Reactions
... react with an accurately and precisely weighed sample of primary standard. Primary standards are ultra-pure solid compounds with high molecular weights and reliable stability. Once a solution is standardized, it may be used as a secondary standard for determining the concentration of other solutions ...
... react with an accurately and precisely weighed sample of primary standard. Primary standards are ultra-pure solid compounds with high molecular weights and reliable stability. Once a solution is standardized, it may be used as a secondary standard for determining the concentration of other solutions ...
theodore l. brown h. eugene lemay, jr. bruce e. bursten catherine j
... system, or transmission in any form or by any means, electronic, mechanical, photocopying, recording, or likewise. To obtain permission(s) to use material from this work, please submit a written request to Pearson Education, Inc., Permissions Department, 1900 E. Lake Ave., Glenview, IL 60025. Many o ...
... system, or transmission in any form or by any means, electronic, mechanical, photocopying, recording, or likewise. To obtain permission(s) to use material from this work, please submit a written request to Pearson Education, Inc., Permissions Department, 1900 E. Lake Ave., Glenview, IL 60025. Many o ...
chemical equation - HCC Learning Web
... • Stoichiometry is the calculation of the quantities of reactants and products involved in a chemical ...
... • Stoichiometry is the calculation of the quantities of reactants and products involved in a chemical ...
www.xtremepapers.net
... Specimen papers for Papers 31/32, 4 and 5 are available on the Teacher Support Site. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, Learning Outcomes have been used throughout. Each part of the syllabus is specified by a br ...
... Specimen papers for Papers 31/32, 4 and 5 are available on the Teacher Support Site. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, Learning Outcomes have been used throughout. Each part of the syllabus is specified by a br ...