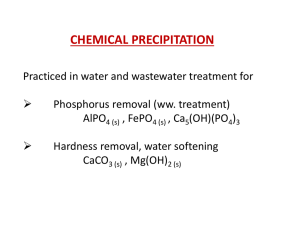
Phosphorus Removal from Wastewater by Chemical Precipitation
... However, these reactions are simple and must be considered in the light of many competing reactions. ...
... However, these reactions are simple and must be considered in the light of many competing reactions. ...
Chapter 4: Aqueous Reactions and Solution Stoichiometry
... with water molecules omitted for clarity. Rank them from strongest to weakest ...
... with water molecules omitted for clarity. Rank them from strongest to weakest ...
study of the contribution of nobel prize winners to the development
... atoms. Major studies have been associated with the excitation and ionization of mercury vapours by strokes of electrons, which formed the content of „FranckHertz experiment‟ (1913). In the experiment between the cathode and the grid they applied potential difference that accelerated electrons collid ...
... atoms. Major studies have been associated with the excitation and ionization of mercury vapours by strokes of electrons, which formed the content of „FranckHertz experiment‟ (1913). In the experiment between the cathode and the grid they applied potential difference that accelerated electrons collid ...
Name: Date: ______ 1. Which of the following is a property of both
... (1) A basis for distinguishing between an element and a compound is whether the substance can be decomposed into other substances using chemical means. (2) Current chemical theory strongly suggests that all naturally occurring elements have been identified. (3) The elements silver, gold, and aluminu ...
... (1) A basis for distinguishing between an element and a compound is whether the substance can be decomposed into other substances using chemical means. (2) Current chemical theory strongly suggests that all naturally occurring elements have been identified. (3) The elements silver, gold, and aluminu ...
Kinetics and Mechanism of Uncatalyzed and Ag (I) Catalyzed
... sulphuric acid medium and sulphato complexes, such as CeSO42+, Ce(SO4)2 and Ce(SO4)32- have been established and quantified [26]. However, cerium (IV) in perchloric acid medium does not indicate complex formation, although Ce4+, Ce(OH)3+, (Ce-O-Ce)6+ and (HOCe-O-CeOH)4+ species of cerium (IV) are we ...
... sulphuric acid medium and sulphato complexes, such as CeSO42+, Ce(SO4)2 and Ce(SO4)32- have been established and quantified [26]. However, cerium (IV) in perchloric acid medium does not indicate complex formation, although Ce4+, Ce(OH)3+, (Ce-O-Ce)6+ and (HOCe-O-CeOH)4+ species of cerium (IV) are we ...
Full Text PDF
... this means that the overlapping of metal and ligand orbitals provides a path by which metal electrons can, and do, escape to a certain extent from 3d-ion towards ligands and molecule boundaries. The effect has been named "nephelauxetic" (expanding cloud, from Greek) [6]. Summing up: β values, which ...
... this means that the overlapping of metal and ligand orbitals provides a path by which metal electrons can, and do, escape to a certain extent from 3d-ion towards ligands and molecule boundaries. The effect has been named "nephelauxetic" (expanding cloud, from Greek) [6]. Summing up: β values, which ...
Chapter 1 Matter and Change
... 1) Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. – Vapor- a substance that is currently a gas, but normally is a liquid or solid at ...
... 1) Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. – Vapor- a substance that is currently a gas, but normally is a liquid or solid at ...
Atoms
... • Each electron shell can hold a specific maximum number of electrons. • The valence electrons are in the outermost electron shell of an atom. Electrons that are not valence electrons are called core electrons. (cont.) © 2004 Key Curriculum Press. ...
... • Each electron shell can hold a specific maximum number of electrons. • The valence electrons are in the outermost electron shell of an atom. Electrons that are not valence electrons are called core electrons. (cont.) © 2004 Key Curriculum Press. ...
1 Course Code– CH1141 Semester – I Credit
... 10. Name a carrier gas used in gas chromatography. 10x1 = 10 marks ...
... 10. Name a carrier gas used in gas chromatography. 10x1 = 10 marks ...
No Slide Title - Cloudfront.net
... • According to the Aufbau principle, an electron occupies the lowest-energy orbital that can receive it. • This means that the electrons do not fill in orbitals in a linear fasion, they fill in following the arrow rule • Atoms want to be in the lowest energy state. This is because higher principle n ...
... • According to the Aufbau principle, an electron occupies the lowest-energy orbital that can receive it. • This means that the electrons do not fill in orbitals in a linear fasion, they fill in following the arrow rule • Atoms want to be in the lowest energy state. This is because higher principle n ...
Synthesis Reaction
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
Document
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
Appendix N CONCENTRATION UNITS
... solutes or mixtures of solutes. Parts per million is also used to describe concentration levels to those who are unfamiliar with the concept of moles. Tap water contains a variety of dissolved impurities, mostly minerals. A simple way of describing the levels of these impurities, often called dissol ...
... solutes or mixtures of solutes. Parts per million is also used to describe concentration levels to those who are unfamiliar with the concept of moles. Tap water contains a variety of dissolved impurities, mostly minerals. A simple way of describing the levels of these impurities, often called dissol ...
The Atomic Molecular Theory
... why these results strongly indicate that the elements carbon and hydrogen are composed of atoms. ...
... why these results strongly indicate that the elements carbon and hydrogen are composed of atoms. ...
ΔH - GCC
... Solution We then replace 2 O2 on the left with O by incorporating the last equation. To do so, we divide the third equation by 2 and reverse its direction. As a result, we must also divide ΔH value by 2 and change its sign. ...
... Solution We then replace 2 O2 on the left with O by incorporating the last equation. To do so, we divide the third equation by 2 and reverse its direction. As a result, we must also divide ΔH value by 2 and change its sign. ...
Chap4 Review - armstrongchemistry
... average mass of the isotopes of that element. Based on this definition, which of these does NOT show the correct atomic mass for an element? A ...
... average mass of the isotopes of that element. Based on this definition, which of these does NOT show the correct atomic mass for an element? A ...
Thermochemistry Exam Review Questions
... D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substance that can react as either a Bronsted-Lowry acid or base? A. amphoteric B. buffer C. indicator ...
... D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substance that can react as either a Bronsted-Lowry acid or base? A. amphoteric B. buffer C. indicator ...
Chemical Reactions
... Lessons 2-4 include a second way to describe chemical reactions. This is accomplished by evaluating the they types of products formed or the oxidation states of the elements involved. There are three types of reactions that we will discuss: Precipitation reactions, Acid / Base Neutralization reactio ...
... Lessons 2-4 include a second way to describe chemical reactions. This is accomplished by evaluating the they types of products formed or the oxidation states of the elements involved. There are three types of reactions that we will discuss: Precipitation reactions, Acid / Base Neutralization reactio ...
(a) From , 2013 General Chemistry I
... the temperature of a sample as it is heated at a constant rate at constant pressure and therefore at a constant rate of increase in enthalpy. -The steeper the slope of a heating curve, the lower the heat capacity. -The horizontal sections correspond to phase changes: melting and boiling. ...
... the temperature of a sample as it is heated at a constant rate at constant pressure and therefore at a constant rate of increase in enthalpy. -The steeper the slope of a heating curve, the lower the heat capacity. -The horizontal sections correspond to phase changes: melting and boiling. ...
Solutions_C19
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...