4.1 Writing and Balancing Chemical Equations
... Precipitation Reactions and Solubility Rules A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displace ...
... Precipitation Reactions and Solubility Rules A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displace ...
C7 Revision Notes 2015
... The –OH in alcohols -89 makes the boiling point higher, more like water. This makes alcohol a liquid at room temperature. Alkanes don’t have the –OH and so are gases at RT ...
... The –OH in alcohols -89 makes the boiling point higher, more like water. This makes alcohol a liquid at room temperature. Alkanes don’t have the –OH and so are gases at RT ...
Summary - Clydebank High School
... 4. ............... der ........................ forces are weak forces of attraction that exists between covalent molecules and also between the atoms of ............................. gases (group 8). .................... der ................... forces are much .................................. tha ...
... 4. ............... der ........................ forces are weak forces of attraction that exists between covalent molecules and also between the atoms of ............................. gases (group 8). .................... der ................... forces are much .................................. tha ...
InorgCh8.2
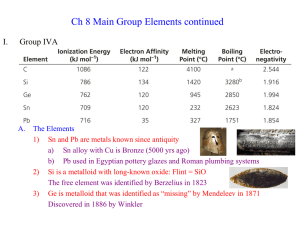
... e) At2 in 1940 during particle collisions (radioactive with ½ life = only 8 hours) 3) Chemistry dominated by ready reduction to Xa) Excellent oxidizing agents (is reduced itself); F2 is best of all elements b) F is the most electronegative element, decreases down the group c) I2 has the highest Lond ...
... e) At2 in 1940 during particle collisions (radioactive with ½ life = only 8 hours) 3) Chemistry dominated by ready reduction to Xa) Excellent oxidizing agents (is reduced itself); F2 is best of all elements b) F is the most electronegative element, decreases down the group c) I2 has the highest Lond ...
4.1 Defining the Atom
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
- Angelo State University
... • If the total mass of a sample of small objects is known, and the average mass of each small object is known, the number of objects in the sample can be determined. ...
... • If the total mass of a sample of small objects is known, and the average mass of each small object is known, the number of objects in the sample can be determined. ...
Revised Syllabus - M. Sc. First Year - Chemistry
... Student with FC grade in a course would be granted credits for that course but not the grade for that course. He/she shall have to clear the concerned course within 1.5 year from appearing for the first time in concerned paper, provided the number of courses with FC and FR grades together is 25% o ...
... Student with FC grade in a course would be granted credits for that course but not the grade for that course. He/she shall have to clear the concerned course within 1.5 year from appearing for the first time in concerned paper, provided the number of courses with FC and FR grades together is 25% o ...
Preview Sample 3
... 9. Understand the nature of the atomic mass scale. 10. Calculate the average atomic mass of an element given the atomic mass and relative abundance of each of its naturally occurring isotopes. 11. Understand the concept of the mole. 12. Use the relationships between Avogadro’s number, moles, molar m ...
... 9. Understand the nature of the atomic mass scale. 10. Calculate the average atomic mass of an element given the atomic mass and relative abundance of each of its naturally occurring isotopes. 11. Understand the concept of the mole. 12. Use the relationships between Avogadro’s number, moles, molar m ...
2.2 the observations that led to an atomic view of matter
... Table 2.1 shows a striking example: soft, silvery sodium metal and yellowgreen, poisonous chlorine gas are very different from the compound they form—white, crystalline sodium chloride, or common table salt! Figure 2.1 Elements, compounds, and mixtures on the atomic scale. The samples depicted here ...
... Table 2.1 shows a striking example: soft, silvery sodium metal and yellowgreen, poisonous chlorine gas are very different from the compound they form—white, crystalline sodium chloride, or common table salt! Figure 2.1 Elements, compounds, and mixtures on the atomic scale. The samples depicted here ...
Stoichiometry1
... The reactant that makes the least amount of product is the limiting reactant. Once you determine the limiting reactant, you should ALWAYS start with it! Be sure to pick a product! You can’t compare to see which is greater and which is lower unless the product is the same! ...
... The reactant that makes the least amount of product is the limiting reactant. Once you determine the limiting reactant, you should ALWAYS start with it! Be sure to pick a product! You can’t compare to see which is greater and which is lower unless the product is the same! ...
Models of the Atom - Fulton County Schools
... Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. ...
... Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. ...
13.0 Redox Reactions PowerPoint
... transferred between entities • The total number of electrons gained in the reduction equals the total number of electrons lost in the oxidation • Reduction is a process in which electrons are gained by an entity • Oxidation is a process in which electrons are lost by an entity • Both reduction and o ...
... transferred between entities • The total number of electrons gained in the reduction equals the total number of electrons lost in the oxidation • Reduction is a process in which electrons are gained by an entity • Oxidation is a process in which electrons are lost by an entity • Both reduction and o ...
Oxidation-Reduction Reactions
... bond called a covalent bond. Even though electrons are not completely transferred between atoms, it is still common for reactions involving molecular compounds to be classified as redox reactions. For example, when hydrogen gas is reacted with oxygen gas, water is formed as the product. 2H2 (g) + O2 ...
... bond called a covalent bond. Even though electrons are not completely transferred between atoms, it is still common for reactions involving molecular compounds to be classified as redox reactions. For example, when hydrogen gas is reacted with oxygen gas, water is formed as the product. 2H2 (g) + O2 ...
Practice Exam 4
... molar entropy. The gases will increase in entropy in the order Ne(g) < Ar(g) < CO2 (g). Ne and Ar are both atoms so they should have less entropy than a molecular substance, which has more complexity. Ar will have a higher entropy than Ne because it has a larger mass and more fundamental particles. ...
... molar entropy. The gases will increase in entropy in the order Ne(g) < Ar(g) < CO2 (g). Ne and Ar are both atoms so they should have less entropy than a molecular substance, which has more complexity. Ar will have a higher entropy than Ne because it has a larger mass and more fundamental particles. ...
H - sintak
... Since both graphite and oxygen are stable allotrophic forms, ∆H°ƒ (C, graphite) and ∆H°ƒ (O2, g) are zero. ∆H°rxn = (1mol) ∆H°ƒ (CO2, g) = -393.5 kJ ∆H°ƒ (CO2, g) = -393.5 kJ/mol ...
... Since both graphite and oxygen are stable allotrophic forms, ∆H°ƒ (C, graphite) and ∆H°ƒ (O2, g) are zero. ∆H°rxn = (1mol) ∆H°ƒ (CO2, g) = -393.5 kJ ∆H°ƒ (CO2, g) = -393.5 kJ/mol ...
Document
... The first term in the target equation for the formation of nitrogen monoxide is one mole of nitrogen gas. We therefore need to double equation (1) so that N2(g) will appear on the reactant side when we add the equations. However, from equation (2) we want 2 mol of NO(g) to appear as a product, so we ...
... The first term in the target equation for the formation of nitrogen monoxide is one mole of nitrogen gas. We therefore need to double equation (1) so that N2(g) will appear on the reactant side when we add the equations. However, from equation (2) we want 2 mol of NO(g) to appear as a product, so we ...
Section 5 – Harry Moseley: Numbering the
... industrial patents in 1997 showed that the majority of the papers cited in the patents were derived from publically supported scientific research. ...
... industrial patents in 1997 showed that the majority of the papers cited in the patents were derived from publically supported scientific research. ...
chemistry
... Base your answers to questions 76 and 77 on the information below. Archimedes (287–212 BC), a Greek inventor and mathematician, made several discoveries important to science today. According to a legend, Hiero, the king of Syracuse, commanded Archimedes to find out if the royal crown was made of go ...
... Base your answers to questions 76 and 77 on the information below. Archimedes (287–212 BC), a Greek inventor and mathematician, made several discoveries important to science today. According to a legend, Hiero, the king of Syracuse, commanded Archimedes to find out if the royal crown was made of go ...
4.3 Distinguishing Among Atoms
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
Outline of chemistry
... central to chemistry, and a great deal of experimental (as well as applied/industrial) chemistry is done without it. A diagram of an atom based on the Rutherford model A chemical reaction is a transformation of some substances into one or more different substances.[39] The ba- The atom is the basic u ...
... central to chemistry, and a great deal of experimental (as well as applied/industrial) chemistry is done without it. A diagram of an atom based on the Rutherford model A chemical reaction is a transformation of some substances into one or more different substances.[39] The ba- The atom is the basic u ...
02_Lecture - WordPress.com
... Elements are represented by a one or two letter symbol. This is the symbol for carbon. All atoms of the same element have the same number of protons, which is called the atomic number, Z. It is written as a subscript BEFORE the symbol. The mass number is the total number of protons and neutron ...
... Elements are represented by a one or two letter symbol. This is the symbol for carbon. All atoms of the same element have the same number of protons, which is called the atomic number, Z. It is written as a subscript BEFORE the symbol. The mass number is the total number of protons and neutron ...
Atomic Models 100
... Answer =In an atom, the central core that contains most of the atom’s mass. Protons and neutrons are located there. Back to Main ...
... Answer =In an atom, the central core that contains most of the atom’s mass. Protons and neutrons are located there. Back to Main ...