www.xtremepapers.net
... Specimen papers for Papers 31/32, 4 and 5 are available on the Teacher Support Site. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, Learning Outcomes have been used throughout. Each part of the syllabus is specified by a br ...
... Specimen papers for Papers 31/32, 4 and 5 are available on the Teacher Support Site. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, Learning Outcomes have been used throughout. Each part of the syllabus is specified by a br ...
B - eko.olunet.org
... composition of impurities/additives are of great importance. For his experiments, Thomas needed KBr with at least 95.0% purity. In order to determine the purity of an available inorganic compound, he weighed out 0.8230 g of KBr and dissolved it in water. Then to the resulting solution, 31.20 cm3 of ...
... composition of impurities/additives are of great importance. For his experiments, Thomas needed KBr with at least 95.0% purity. In order to determine the purity of an available inorganic compound, he weighed out 0.8230 g of KBr and dissolved it in water. Then to the resulting solution, 31.20 cm3 of ...
chemistry
... (i) It failed to explain how atoms of different elements differ from each other. (ii) It failed to explain how and why atoms of elements combine with each other to form compound or molecules. (iii) It failed to explain the nature of forces that bind together different atoms in a molecule. (iv) It di ...
... (i) It failed to explain how atoms of different elements differ from each other. (ii) It failed to explain how and why atoms of elements combine with each other to form compound or molecules. (iii) It failed to explain the nature of forces that bind together different atoms in a molecule. (iv) It di ...
Standard - Santee Education Complex
... acid that your stomach uses to digest food.) Table salt How can this be if both materials have chlorine in them? The chemical bonding that takes (NaCl) place in NaCl is different than that in HCl. This gives NaCl and HCl very different structures, appearances, and properties.What other differences a ...
... acid that your stomach uses to digest food.) Table salt How can this be if both materials have chlorine in them? The chemical bonding that takes (NaCl) place in NaCl is different than that in HCl. This gives NaCl and HCl very different structures, appearances, and properties.What other differences a ...
spring semester review
... a) HNO2 + K+ + OH- ---> KNO2 + H2O b) HNO2 + H2O ----> NO2- + H3O+ c) HNO2 + KOH ----> K+ + NO2- + H2O d) HNO2 + OH- ----> NO2- + H2O e) H+ + OH- ----> H2O 34. A solution of ammonia is titrated with hydrochloric acid. At the equivalence point, phenolphthalein will be what color? a) colorless b) pink ...
... a) HNO2 + K+ + OH- ---> KNO2 + H2O b) HNO2 + H2O ----> NO2- + H3O+ c) HNO2 + KOH ----> K+ + NO2- + H2O d) HNO2 + OH- ----> NO2- + H2O e) H+ + OH- ----> H2O 34. A solution of ammonia is titrated with hydrochloric acid. At the equivalence point, phenolphthalein will be what color? a) colorless b) pink ...
Chapter 3
... It is important to know the mass of the atoms especially for the lab work. However; atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it ...
... It is important to know the mass of the atoms especially for the lab work. However; atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it ...
Chapter 6 Electronic Structure of Atoms
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
MT 3 Practice
... [A] It is spontaneous at relatively low temperatures only. [B] It is spontaneous at all temperatures. [C] It is nonspontaneous at all temperatures. [D] It is spontaneous at relatively high temperatures only. 13. What is the minimum temperature required for the spontaneous conversion of CCl4(l) to CC ...
... [A] It is spontaneous at relatively low temperatures only. [B] It is spontaneous at all temperatures. [C] It is nonspontaneous at all temperatures. [D] It is spontaneous at relatively high temperatures only. 13. What is the minimum temperature required for the spontaneous conversion of CCl4(l) to CC ...
Atom Building - Campbell County Schools
... • Neils Bohr hypothesized that the atom was like a tiny solar system, with electrons circling the nucleus in well defined orbits like planets • The speculated paths of the electrons were called orbitals, shells, or energy ...
... • Neils Bohr hypothesized that the atom was like a tiny solar system, with electrons circling the nucleus in well defined orbits like planets • The speculated paths of the electrons were called orbitals, shells, or energy ...
Unit F325 - Equilibria, energetics and elements - High band
... candidate. The final answer should have been given to 3 significant figures as all values in the question are to this precision. The answer of 0.8 against the actual calculated value of 0.848 introduces a 6% rounding error. This is a basic error and loss of marks such as this could prove costly for ...
... candidate. The final answer should have been given to 3 significant figures as all values in the question are to this precision. The answer of 0.8 against the actual calculated value of 0.848 introduces a 6% rounding error. This is a basic error and loss of marks such as this could prove costly for ...
Chapter 3 Chemical Reactions and Reaction Stoichiometry
... Ø %-yield can be less than 100% if, among other reasons, the reaction achieves equilibrium before coming to completion or if some reactants and/or products are physically lost going from one experimental step to another. Ø %-yield can be greater than 100% if the product contains impurities. Thes ...
... Ø %-yield can be less than 100% if, among other reasons, the reaction achieves equilibrium before coming to completion or if some reactants and/or products are physically lost going from one experimental step to another. Ø %-yield can be greater than 100% if the product contains impurities. Thes ...
Chapter 3 Chemical Compounds
... named using the element name plus the word ‘ion’. So, Na+ is called the sodium ion and Ca2+ is called the calcium ion. For main group metals (metals in groups with an ‘A’ in their column number), the positive charge of the metal cation is equal to the metal’s column number. So, alkali metal ions (g ...
... named using the element name plus the word ‘ion’. So, Na+ is called the sodium ion and Ca2+ is called the calcium ion. For main group metals (metals in groups with an ‘A’ in their column number), the positive charge of the metal cation is equal to the metal’s column number. So, alkali metal ions (g ...
Chemistry II Honors – Unit 3 Study Guide
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.65e13 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each red b ...
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.65e13 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each red b ...
Chapter 2 Atoms, Molecules, and Ions
... Dalton’s Postulates Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. (As far as Dalton knew, they couldn’t be changed at all). ...
... Dalton’s Postulates Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. (As far as Dalton knew, they couldn’t be changed at all). ...
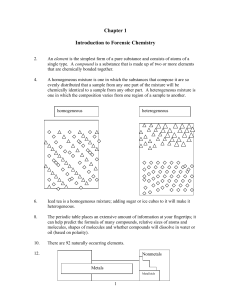
Chapter 1 Introduction to Forensic Chemistry
... carbon dioxide and hydrogen-containing compounds then produce water as a result. Neutralization reactions occur when an acid and a base react to form a salt and water. Redox reactions occur when one substance gains electrons (decreases its oxidation state) and another substance loses electrons (incr ...
... carbon dioxide and hydrogen-containing compounds then produce water as a result. Neutralization reactions occur when an acid and a base react to form a salt and water. Redox reactions occur when one substance gains electrons (decreases its oxidation state) and another substance loses electrons (incr ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Principles of Chemistry: A Molecular Approach, 1st Ed. ...
... Principles of Chemistry: A Molecular Approach, 1st Ed. ...
Distinguishing Among Atoms - Chapter 4 Section 3 Student Guided
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
Part One: Mass and Moles of Substance A. Molecular Mass and
... So molecular formula is C8H8O2 12. The Law of Multiple Proportions (revisited) = when two elements, A and B, form more than one compound together, the amount of B combining with a given amount of A in each compound forms a whole number ratio: amt B combining w/A in cmpd 1 = ratio of whole numbers am ...
... So molecular formula is C8H8O2 12. The Law of Multiple Proportions (revisited) = when two elements, A and B, form more than one compound together, the amount of B combining with a given amount of A in each compound forms a whole number ratio: amt B combining w/A in cmpd 1 = ratio of whole numbers am ...
4.3 Distinguishing Among Atoms
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
Atom
... The atomic number and mass number are needed to determine an atom s composition. The atomic number gives the number of protons, which equals the number of electrons. The number of neutrons is the difference between the mass number and the atomic number. ...
... The atomic number and mass number are needed to determine an atom s composition. The atomic number gives the number of protons, which equals the number of electrons. The number of neutrons is the difference between the mass number and the atomic number. ...
4.3 Distinguishing Among Atoms - Miami Beach Senior High School
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
Document
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
... Such data about the actual masses of individual atoms can provide useful information, but in general these values are inconveniently small and impractical to work with. • Instead, it is more useful to compare the relative masses of atoms using a reference isotope as a standard. • The reference isoto ...
DEPARTMENT OF CHEMISTRY AND CHEMICAL TECHNOLOGY
... Print your name: _____________________________________________ ...
... Print your name: _____________________________________________ ...
Document
... of chemical potential energy. This stored energy is related to the relative positions of particles and the strengths of the bonds between them. Potential energy is stored or released as the positions of the particles change, just as it is when a spring is stretched and then released. As bonds break ...
... of chemical potential energy. This stored energy is related to the relative positions of particles and the strengths of the bonds between them. Potential energy is stored or released as the positions of the particles change, just as it is when a spring is stretched and then released. As bonds break ...