California Standards Practice - Student Edition
... alkaline earth metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ions and atoms. d. Students know how to use the periodic table to determine the number of electrons available for bonding. e. Students know the nucleus of the atom is much smaller t ...
... alkaline earth metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ions and atoms. d. Students know how to use the periodic table to determine the number of electrons available for bonding. e. Students know the nucleus of the atom is much smaller t ...
- University Of Nigeria Nsukka
... electron from one atom t o the other because the fluorine atom has a much greater affinity, a much greater propensity for electrons. O r , t o use the chemist's language, the fluorine atom is muchlmore electronegative than the d i u m atom. In the second reaction, i,e. (ii), the two fluorine atoms a ...
... electron from one atom t o the other because the fluorine atom has a much greater affinity, a much greater propensity for electrons. O r , t o use the chemist's language, the fluorine atom is muchlmore electronegative than the d i u m atom. In the second reaction, i,e. (ii), the two fluorine atoms a ...
L-11 Chemical thermodynamics
... You know that hot tea/milk (let us call it a system) kept in a stoppered thermos flask remains hot for a couple of hours. If this flask is made of perfect insulating material, then there would be no exchange of matter or energy between the system and the surroundings. We call such a system an isolat ...
... You know that hot tea/milk (let us call it a system) kept in a stoppered thermos flask remains hot for a couple of hours. If this flask is made of perfect insulating material, then there would be no exchange of matter or energy between the system and the surroundings. We call such a system an isolat ...
SAMPLE QUESTION PAPER CHEMISTRY (313)
... (ii) An electrophile is positively charged species It is election setting, It attacks at position of high density. Examples H+ NO+2 , Ag+ (iii) Nucleophile is a negatively charged species. It is nucleus seeking. It attacks a position of low election density examples OH– NO2− etc. (iii) The property ...
... (ii) An electrophile is positively charged species It is election setting, It attacks at position of high density. Examples H+ NO+2 , Ag+ (iii) Nucleophile is a negatively charged species. It is nucleus seeking. It attacks a position of low election density examples OH– NO2− etc. (iii) The property ...
File
... – write down the charge on each particle, the mass of each particle, and where they are found in the atom. Electron = neg. charge found outside nucleus, almost zero mass Protons = pos. charge, in nucleus, 1 amu Neutron = no charge, in nucleus, 1 amu ...
... – write down the charge on each particle, the mass of each particle, and where they are found in the atom. Electron = neg. charge found outside nucleus, almost zero mass Protons = pos. charge, in nucleus, 1 amu Neutron = no charge, in nucleus, 1 amu ...
Honors Chemistry Unit 02
... – All atoms of the same element have the same mass (not quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms chang ...
... – All atoms of the same element have the same mass (not quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms chang ...
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
CHEM102 Chemistry II Spring 11-12 Mid
... 28) Which of the following can serve as the solvent in a solution? 28) ______ A) a liquid B) a gas C) a solid D) a mixture of comingled liquids E) all of the above 29) If the concentration of H3O+ is 3.5 × 10-3 M, the concentration of OH- is ________ M. 29) ______ A) 3.5 × 10-11 B) 1.0 × 10-12 C) 2. ...
... 28) Which of the following can serve as the solvent in a solution? 28) ______ A) a liquid B) a gas C) a solid D) a mixture of comingled liquids E) all of the above 29) If the concentration of H3O+ is 3.5 × 10-3 M, the concentration of OH- is ________ M. 29) ______ A) 3.5 × 10-11 B) 1.0 × 10-12 C) 2. ...
Unit 2 Powerpoint Notes
... The Mole • A mole (mol) is the amount of a substance that contains as many particles as there are atoms in exactly 12 g of carbon-12. • It is a counting unit, similar to a dozen. In a dozen, there are 12 things. In a mole, there are 6.02 x 1023 things. ...
... The Mole • A mole (mol) is the amount of a substance that contains as many particles as there are atoms in exactly 12 g of carbon-12. • It is a counting unit, similar to a dozen. In a dozen, there are 12 things. In a mole, there are 6.02 x 1023 things. ...
2.1 Atomic Theory of Matter
... for the most stable ion of barium and the most stable ion of oxygen. Practice Exercise 12 Although it is helpful to know that many ions have the electron arrangement of a noble gas, many elements, especially among the metals, form ions that do not have a noble-bas electron arrangement. Use the perio ...
... for the most stable ion of barium and the most stable ion of oxygen. Practice Exercise 12 Although it is helpful to know that many ions have the electron arrangement of a noble gas, many elements, especially among the metals, form ions that do not have a noble-bas electron arrangement. Use the perio ...
DRAFT AP® CHEMISTRY 2005 SCORING GUIDELINES
... Should accept oxygen gas causes splint to RE_IGNITE. The popping sound presumed the accessibility of oxygen, which is not explicit in the directions. What to do if a student claims that the splint will be extinguished? I would give the credit. Also, would accept ‘re-ignite’ for oxygen. I have a prob ...
... Should accept oxygen gas causes splint to RE_IGNITE. The popping sound presumed the accessibility of oxygen, which is not explicit in the directions. What to do if a student claims that the splint will be extinguished? I would give the credit. Also, would accept ‘re-ignite’ for oxygen. I have a prob ...
Stoichiometry We compare all other elements to the known mass of
... Calculating empirical and molecular formulas: empirical formulas represent the simplest or smallest m o l a r ratio of elements within a compound while molecular formulas represent the actual numbers of elements within a compound. The empirical mass is the least common multiple of the molar mass. Ex ...
... Calculating empirical and molecular formulas: empirical formulas represent the simplest or smallest m o l a r ratio of elements within a compound while molecular formulas represent the actual numbers of elements within a compound. The empirical mass is the least common multiple of the molar mass. Ex ...
Thermodynamics - WordPress.com
... 40. Entropy of perfectly crystalline solid at absolute zero is zero. 41. A process which can be reversed at any instant of time by increasing the opposing force by an infinitesimal amount. 42. A process which is carried out rapidly so that the system does not get a chance to attain equilibrium. 43. ...
... 40. Entropy of perfectly crystalline solid at absolute zero is zero. 41. A process which can be reversed at any instant of time by increasing the opposing force by an infinitesimal amount. 42. A process which is carried out rapidly so that the system does not get a chance to attain equilibrium. 43. ...
Honors Chemistry I
... 2) Count the total number of atoms of each element on each side of the equation a. If there is an imbalance of an atom, start planning a strategy to bring balance to the equation b. You will use coefficients to balance equations. 3) It’s best to focus on balancing one element at a time, usually star ...
... 2) Count the total number of atoms of each element on each side of the equation a. If there is an imbalance of an atom, start planning a strategy to bring balance to the equation b. You will use coefficients to balance equations. 3) It’s best to focus on balancing one element at a time, usually star ...
Atomic Theory PPT
... charge equal in magnitude to the electron’s negative charge. However, it is about 1800 times heavier than the electron. Every element has a specific number of protons. Change the number of protons and you have a new element. Imagine the possibilities! Rutherford had theorized that there must be anot ...
... charge equal in magnitude to the electron’s negative charge. However, it is about 1800 times heavier than the electron. Every element has a specific number of protons. Change the number of protons and you have a new element. Imagine the possibilities! Rutherford had theorized that there must be anot ...
ChemChapter_7sec1_and_section2[1]FORMULA
... examples: all atoms in sodium, Na, oxygen, O2, phosphorus, P4, and sulfur, S8, have oxidation numbers of zero. 2. The more-electronegative element in a binary compound is assigned a negative number equal to the charge it would have as an anion. Likewise for the less-electronegative element. 3. Fluor ...
... examples: all atoms in sodium, Na, oxygen, O2, phosphorus, P4, and sulfur, S8, have oxidation numbers of zero. 2. The more-electronegative element in a binary compound is assigned a negative number equal to the charge it would have as an anion. Likewise for the less-electronegative element. 3. Fluor ...
FREE Sample Here
... B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einstein. D) Dalton, and widely accepted within a few decades. Answer: A Topic: Section 2.1 Conservation of Mass and the Law of Definite Proportions 2) The observation that 15.0 g of ...
... B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einstein. D) Dalton, and widely accepted within a few decades. Answer: A Topic: Section 2.1 Conservation of Mass and the Law of Definite Proportions 2) The observation that 15.0 g of ...
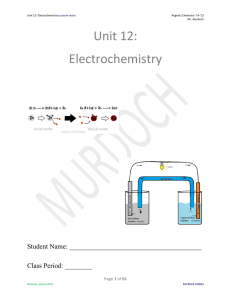
Unit 12: Electrochemistry
... ionization energy; they (and what it means for the can easily lose electrons element) - Table S when energy is added ...
... ionization energy; they (and what it means for the can easily lose electrons element) - Table S when energy is added ...
WIPO IPC: Internet Publication
... and a non-chemical part or aspect, the general rule is that the chemical part or aspect is covered by section C. In some of these cases, the chemical part or aspect brings with it a non-chemical one, even though purely mechanical, because this latter aspect either is essential to the operation or tr ...
... and a non-chemical part or aspect, the general rule is that the chemical part or aspect is covered by section C. In some of these cases, the chemical part or aspect brings with it a non-chemical one, even though purely mechanical, because this latter aspect either is essential to the operation or tr ...
Accurate van der Waals interactions from groundstate
... 2) Ab initio reference freeatom C6 coefficients (Chu and Dalgarno). 3) Accurate combination rule for heteronuclear coefficients. 4) Atominamolecule/solid polarizability from Hirshfeld partitioning of the electron density (Becke and Johnson). 5) C6 = C6[n(r)]: C6 becomes a functional of the elec ...
... 2) Ab initio reference freeatom C6 coefficients (Chu and Dalgarno). 3) Accurate combination rule for heteronuclear coefficients. 4) Atominamolecule/solid polarizability from Hirshfeld partitioning of the electron density (Becke and Johnson). 5) C6 = C6[n(r)]: C6 becomes a functional of the elec ...
chemistry -- questions -
... a) The first energy level will have 8 and the second will have 7. b) The first energy level will have 2, the second will have 8, and the third will have 5. c) The first energy level will have 2 and the second will have 13. d) The first, second, and third energy levels will each have 5 electrons. e) ...
... a) The first energy level will have 8 and the second will have 7. b) The first energy level will have 2, the second will have 8, and the third will have 5. c) The first energy level will have 2 and the second will have 13. d) The first, second, and third energy levels will each have 5 electrons. e) ...
Chapter 3: Calculations with Chemical Formulas
... it is soluble in water. According to the chart, most compounds that contain carbonate, CO32−, are insoluble. CaCO3 is not one of the exceptions, so it is insoluble in water. Mercuric nitrate or Hg(NO3)2 is soluble because all forms of nitrates are soluble. AgCl is insoluble. According to the chart, ...
... it is soluble in water. According to the chart, most compounds that contain carbonate, CO32−, are insoluble. CaCO3 is not one of the exceptions, so it is insoluble in water. Mercuric nitrate or Hg(NO3)2 is soluble because all forms of nitrates are soluble. AgCl is insoluble. According to the chart, ...
















![ChemChapter_7sec1_and_section2[1]FORMULA](http://s1.studyres.com/store/data/000546743_1-278f96ccbbfd49e292510ec017e27124-300x300.png)