Chem 1202 - LSU Department of Chemistry
... conversion of all forms of energy The First Law of Thermodynamics Energy cannot be created or destroyed; it can be moved from one place to another, and it can be converted from one form to another. Thermodynamics is the set of rules that govern • the movement of energy from one place to ...
... conversion of all forms of energy The First Law of Thermodynamics Energy cannot be created or destroyed; it can be moved from one place to another, and it can be converted from one form to another. Thermodynamics is the set of rules that govern • the movement of energy from one place to ...
CHAP 3.pmd - eVirtualGuru
... element will combine with the atom(s) of another element to for m a chemical compound. The valency of the atom of an element can be thought of as hands or arms of that atom. Human beings have two arms and an octopus has eight. If one octopus has to catch hold of a few people in such a manner that al ...
... element will combine with the atom(s) of another element to for m a chemical compound. The valency of the atom of an element can be thought of as hands or arms of that atom. Human beings have two arms and an octopus has eight. If one octopus has to catch hold of a few people in such a manner that al ...
molecular orbital and valence bond theory explained (hopefully)
... nuclei. Both nuclei are attracted to the electrons between them. The energy of a bonding molecular orbital is lower than the energy of the uncombined atomic orbitals. An antibonding molecular orbital (designated with an *) occurs when the electron density of the orbital is concentrated in region ...
... nuclei. Both nuclei are attracted to the electrons between them. The energy of a bonding molecular orbital is lower than the energy of the uncombined atomic orbitals. An antibonding molecular orbital (designated with an *) occurs when the electron density of the orbital is concentrated in region ...
File
... Equations must be balanced – have same number of each kind of atom in reactants and products, since atoms are not created or destroyed in chemical reactions (law of conservation of mass) 2 Na + 2 H2O 2NaOH + H2, balanced 2, 2, 2, (1) are stoichiometric coefficients - coefficients are relative numb ...
... Equations must be balanced – have same number of each kind of atom in reactants and products, since atoms are not created or destroyed in chemical reactions (law of conservation of mass) 2 Na + 2 H2O 2NaOH + H2, balanced 2, 2, 2, (1) are stoichiometric coefficients - coefficients are relative numb ...
GCE Getting Started - Edexcel
... You will need to know: • the order of filling • how many electrons in each orbital. If you are given the atomic number, you should be able to show how the electrons are arranged in their orbitals: s, p and d. You should also be able to represent these using ‘electrons in boxes’. From KS4, know the r ...
... You will need to know: • the order of filling • how many electrons in each orbital. If you are given the atomic number, you should be able to show how the electrons are arranged in their orbitals: s, p and d. You should also be able to represent these using ‘electrons in boxes’. From KS4, know the r ...
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED
... Because they are so small and are moving so fast, electrons have no defined position. Their location is best described by wave mechanics (i.e. a threedimensional wave) and a wave equation called the Schrödinger equation. Solutions of the Schrödinger equation are called wave functions and are repre ...
... Because they are so small and are moving so fast, electrons have no defined position. Their location is best described by wave mechanics (i.e. a threedimensional wave) and a wave equation called the Schrödinger equation. Solutions of the Schrödinger equation are called wave functions and are repre ...
Quantum Chemistry Predicts Multiply Bonded Diuranium
... calculations with the DKH Hamiltonian.12-13 For uranium, a primitive set 26s23p17d13f5g was contracted to: 9s8p6d4f2g,14 while basis sets of TZP quality were used for H, C, O, and Cl. For atoms as heavy as uranium, it is necessary to include relativistic effects. This was done using the second-order ...
... calculations with the DKH Hamiltonian.12-13 For uranium, a primitive set 26s23p17d13f5g was contracted to: 9s8p6d4f2g,14 while basis sets of TZP quality were used for H, C, O, and Cl. For atoms as heavy as uranium, it is necessary to include relativistic effects. This was done using the second-order ...
Energy and Energy Changes Heat Transfer and The Measurement
... • Example 15-1: When 3.425 kJ of heat is added to a calorimeter containing 50.00 g of water the temperature rises from 24.00oC to 36.54oC. Calculate the heat capacity of the calorimeter in J/oC. The specific heat of water is 4.184 J/g oC. • This is a four part calculation. ...
... • Example 15-1: When 3.425 kJ of heat is added to a calorimeter containing 50.00 g of water the temperature rises from 24.00oC to 36.54oC. Calculate the heat capacity of the calorimeter in J/oC. The specific heat of water is 4.184 J/g oC. • This is a four part calculation. ...
chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Atoms. Molecules, and Ions
... Covalent Bonding (Molecules): The most common type of chemical bond is formed when two atoms share some of their electrons. ...
... Covalent Bonding (Molecules): The most common type of chemical bond is formed when two atoms share some of their electrons. ...
Program Review - Austin Community College
... We envision improved safety measures within our labs. Standard safety and emergency procedures that are used by staff, instructors, and students at all campuses are major priorities. We hope to have functional safety equipment such as eyewashes, showers, and hoods at all campuses. We will keep our c ...
... We envision improved safety measures within our labs. Standard safety and emergency procedures that are used by staff, instructors, and students at all campuses are major priorities. We hope to have functional safety equipment such as eyewashes, showers, and hoods at all campuses. We will keep our c ...
Electron configuration
... • since light has both wave and particle characteristics, an electron might also exhibit these characteristics ...
... • since light has both wave and particle characteristics, an electron might also exhibit these characteristics ...
"Differential cross sections for positron-xenon elastic scattering" Phys. Rev. A 73 (2006) 064702. J. P. Marler, C. M. Surko, R. P. McEachran, and A. D. Stauffer (PDF)
... Experimental studies of positron scattering from atoms and molecules have lagged behind electron scattering because of the difficulties involved in creating sufficiently monoenergetic and intense beams of positrons to carry out such measurements. However, with the recent development of efficient pos ...
... Experimental studies of positron scattering from atoms and molecules have lagged behind electron scattering because of the difficulties involved in creating sufficiently monoenergetic and intense beams of positrons to carry out such measurements. However, with the recent development of efficient pos ...
chemistry (9189)
... have a greater descriptive content. Similarly, choice of option may also be influenced by other subjects being studied alongside chemistry. This deliberate variety is seen as a virtue of the complete syllabus. Notwithstanding the different balances within the options to descriptive/quantitative aspe ...
... have a greater descriptive content. Similarly, choice of option may also be influenced by other subjects being studied alongside chemistry. This deliberate variety is seen as a virtue of the complete syllabus. Notwithstanding the different balances within the options to descriptive/quantitative aspe ...
Block 1 - cloudfront.net
... • Number of wheels = ? wheels The desired conversion is tricycles (FSW3HP,) wheels (W). The balanced equation tells you that each tricycle has three wheels, or 1 FSW3HP2 = 3 W. The problem can be solved by using the proper conversion factor derived from this expression. Calculate Solve for the unk ...
... • Number of wheels = ? wheels The desired conversion is tricycles (FSW3HP,) wheels (W). The balanced equation tells you that each tricycle has three wheels, or 1 FSW3HP2 = 3 W. The problem can be solved by using the proper conversion factor derived from this expression. Calculate Solve for the unk ...
Chapter 3 Stoichiometry: Calculations with Chemical
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
MIDDLE COLLEGE HIGH SCHOOL
... The rate of this reaction can be increased by (1) formation of a precipitate using 5.0 grams of powdered zinc instead of a (2) formation of a gas 5.0-gram strip of zinc because the powdered (3) effective collisions between reacting zinc has particles (1) lower kinetic energy (4) addition of a cataly ...
... The rate of this reaction can be increased by (1) formation of a precipitate using 5.0 grams of powdered zinc instead of a (2) formation of a gas 5.0-gram strip of zinc because the powdered (3) effective collisions between reacting zinc has particles (1) lower kinetic energy (4) addition of a cataly ...
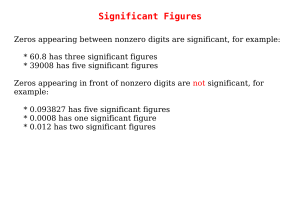
Significant Figures
... to the tens place. Of the two, the least accurate is the hundreds place. The answer should not have significant digits past the hundreds place. ...
... to the tens place. Of the two, the least accurate is the hundreds place. The answer should not have significant digits past the hundreds place. ...
Summer Assignment: Some Review / Basic Prep
... Group 15 metal cations = + 3 commonly (Bi may also be +5) b) Transition metals are those found in groups 3 – 11, (12) i) As a rule, transition metals may have cations of multiple positive oxidation states. This fact is the reason for including the exact whole number oxidation state in the names of i ...
... Group 15 metal cations = + 3 commonly (Bi may also be +5) b) Transition metals are those found in groups 3 – 11, (12) i) As a rule, transition metals may have cations of multiple positive oxidation states. This fact is the reason for including the exact whole number oxidation state in the names of i ...
Unit #8 - consumerchem
... : in the presence of a catalyst. i) Catalyst - 1. a substance that speeds up a chemical reaction without being used up. 2. Lowers the activation energy. 3. Enzymes are biological catalysts. ...
... : in the presence of a catalyst. i) Catalyst - 1. a substance that speeds up a chemical reaction without being used up. 2. Lowers the activation energy. 3. Enzymes are biological catalysts. ...
Atomic Mass - HCC Learning Web
... consumed and produced in chemical reactions. In macroworld, we can count objects by weighing assuming that – Objects behave as though they were all identical – We know the average mass of the objects ...
... consumed and produced in chemical reactions. In macroworld, we can count objects by weighing assuming that – Objects behave as though they were all identical – We know the average mass of the objects ...