Balancing Chemical Equations
... Because of the law of conservation of matter, these equations must be balanced. In other words, the number of atoms of each element must be conserved. For example, look at the reaction of hydrogen and oxygen to produce water: H2 + O2 → H2O. The reactant side of the equation contains two atoms of hyd ...
... Because of the law of conservation of matter, these equations must be balanced. In other words, the number of atoms of each element must be conserved. For example, look at the reaction of hydrogen and oxygen to produce water: H2 + O2 → H2O. The reactant side of the equation contains two atoms of hyd ...
100 Problems and Exercises in Organometallic Chemistry Anil J. Elias
... 30. The reaction of Mo(CO)6 with dicyclopentadiene (C10H12) under microwave conditions yields a stable compound A with the empirical formula C8H5O3Mo along with evolution of CO and H2 gas. The infrared spectrum of this compound gives peaks in the range of 1859-1960 cm-1. Compound A on refluxing in t ...
... 30. The reaction of Mo(CO)6 with dicyclopentadiene (C10H12) under microwave conditions yields a stable compound A with the empirical formula C8H5O3Mo along with evolution of CO and H2 gas. The infrared spectrum of this compound gives peaks in the range of 1859-1960 cm-1. Compound A on refluxing in t ...
23. Oxidation and Reduction
... This is not new to you. You worked with this concept back in Chapter 14. For example, what is the oxidation number of the Mn atom in the ion MnO41-? The total of the oxidation numbers of the atoms in this ion must must equal the charge on the ion, which in this case is -1. Rule 5 in section 23.1 tel ...
... This is not new to you. You worked with this concept back in Chapter 14. For example, what is the oxidation number of the Mn atom in the ion MnO41-? The total of the oxidation numbers of the atoms in this ion must must equal the charge on the ion, which in this case is -1. Rule 5 in section 23.1 tel ...
153KB PDF - Clydeview Academy
... Do not change any of these details. 4 If any of this information is wrong, tell the Invigilator immediately. 5 If this information is correct, print your name and seat number in the boxes provided. 6 The answer to each question is either A, B, C or D. Decide what your answer is, then, using your pen ...
... Do not change any of these details. 4 If any of this information is wrong, tell the Invigilator immediately. 5 If this information is correct, print your name and seat number in the boxes provided. 6 The answer to each question is either A, B, C or D. Decide what your answer is, then, using your pen ...
Chapter 6: Moles, Molar Mass, Percent Composition and Formulas
... b) That’s 602 billion groups of a trillion! c) Let’s just do an example with paper clips. d) If you have a mole of paper clips and made them into a chain, how many times could you go to the moon and back with your chain? (You don’t need to do this) ...
... b) That’s 602 billion groups of a trillion! c) Let’s just do an example with paper clips. d) If you have a mole of paper clips and made them into a chain, how many times could you go to the moon and back with your chain? (You don’t need to do this) ...
WIPO IPC: Internet Publication
... In still other cases, the pure chemical aspect is covered by section C and the applied chemical aspect by another section, such as A, B or F, e.g., the use of a substance or composition for: treatment of plants or animals, covered by subclass A01N; foodstuffs, covered by class A23; ammunition ...
... In still other cases, the pure chemical aspect is covered by section C and the applied chemical aspect by another section, such as A, B or F, e.g., the use of a substance or composition for: treatment of plants or animals, covered by subclass A01N; foodstuffs, covered by class A23; ammunition ...
11 myp covalent bonding
... energetically unstable and therefore they react with other elements to be stable. That atoms of elements, apart from the noble gases, try to attain a stable state by – either losing electrons, or – gaining electrons, or – sharing electrons. • so that they have a completely filled valence (outer) she ...
... energetically unstable and therefore they react with other elements to be stable. That atoms of elements, apart from the noble gases, try to attain a stable state by – either losing electrons, or – gaining electrons, or – sharing electrons. • so that they have a completely filled valence (outer) she ...
chapter 4 - reactions in solution
... o moderates the Earth’s temperature; It is widely used in industrial cooling system, power plants and automobile engines; o o ...
... o moderates the Earth’s temperature; It is widely used in industrial cooling system, power plants and automobile engines; o o ...
1.ThermoStudentNotes
... A student built a simple calorimeter with a 25.0 g tin can and 150 mL of water. Calculate the molar enthalpy of combustion of ethanol in kJ/mol if 0.166 g of this fuel increased the temperature of the calorimeter by 7.00C. Remember to include not only the heat gained by the water but also by the ca ...
... A student built a simple calorimeter with a 25.0 g tin can and 150 mL of water. Calculate the molar enthalpy of combustion of ethanol in kJ/mol if 0.166 g of this fuel increased the temperature of the calorimeter by 7.00C. Remember to include not only the heat gained by the water but also by the ca ...
Dear Students, Welcome to AP Chemistry, a little early. We will have
... assessed on the APC Exam but is assessed on the SAT Chemistry Subject Test 2 so I have included some information on this concept. 3. Read the Chapter 3 notes provided. Complete problems from Hwk 1.3. We will review this section extensively, as stoichiometry is a critical component of APC! The advanc ...
... assessed on the APC Exam but is assessed on the SAT Chemistry Subject Test 2 so I have included some information on this concept. 3. Read the Chapter 3 notes provided. Complete problems from Hwk 1.3. We will review this section extensively, as stoichiometry is a critical component of APC! The advanc ...
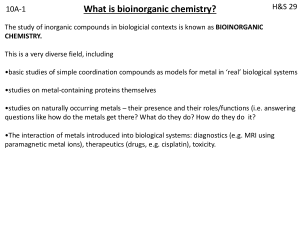
bioinorganic 1
... Electron transfer normally involves movement of one electron at a time (the ultimate use is usually coupled to bond making-breaking). Few naturally occurring organic substrates can do this. Transition metals are excellent for electron transfer because they can adopt more than one oxidation state. Ir ...
... Electron transfer normally involves movement of one electron at a time (the ultimate use is usually coupled to bond making-breaking). Few naturally occurring organic substrates can do this. Transition metals are excellent for electron transfer because they can adopt more than one oxidation state. Ir ...
Packet 4
... solvent, usually water). The concentration of a solution, can be measured in terms of the number of grams of the solute (solid) that has been dissolved in a particular volume of the solvent (usually water), or in terms of the number of moles of the solute in a particular volume of the solvent. Typic ...
... solvent, usually water). The concentration of a solution, can be measured in terms of the number of grams of the solute (solid) that has been dissolved in a particular volume of the solvent (usually water), or in terms of the number of moles of the solute in a particular volume of the solvent. Typic ...
Deuterium fractionation of methylamine through atomic grain
... Deuterium fractionation of methylamine through atomic grain-surface reactions at low temperatures: implication for the possible D/H ratio in molecular clouds Y. Oba, T. Chigai, N. Watanabe, and A. Kouchi Institute of Low Temperature Science, Hokkaido University, Japan Interstellar methylamine (CH3NH ...
... Deuterium fractionation of methylamine through atomic grain-surface reactions at low temperatures: implication for the possible D/H ratio in molecular clouds Y. Oba, T. Chigai, N. Watanabe, and A. Kouchi Institute of Low Temperature Science, Hokkaido University, Japan Interstellar methylamine (CH3NH ...
Differentiated Chemistry Worksheet and Laboratory
... Explain what happens when the electron of a hydrogen atom changes from a 2s orbital to a 5s orbital. ...
... Explain what happens when the electron of a hydrogen atom changes from a 2s orbital to a 5s orbital. ...
CHAPTER 19
... for a few minutes—long enough for the reaction to occur— and then rinsed away with tap water, the smell will disappear. The formula does not bleach or cause any other negative side effects. Mr. Krebaum does have one warning: Mix the formula just before using it, because the mixture breaks down quick ...
... for a few minutes—long enough for the reaction to occur— and then rinsed away with tap water, the smell will disappear. The formula does not bleach or cause any other negative side effects. Mr. Krebaum does have one warning: Mix the formula just before using it, because the mixture breaks down quick ...
No Slide Title
... Bellringer Are you familiar with some of the elements on the periodic table? There are probably many more elements that you have never heard of before. You may be surprised to learn that even though you have never heard of a certain element before, by looking at the periodic table, you can guess som ...
... Bellringer Are you familiar with some of the elements on the periodic table? There are probably many more elements that you have never heard of before. You may be surprised to learn that even though you have never heard of a certain element before, by looking at the periodic table, you can guess som ...
Review Unit: Chemistry Review
... physically before it is considered safe. Rocks and minerals form the foundation of the non-living environment. For example, magnesium carbonate from the Rocky Mountains becomes a main component of sidewalks in our bustling cities. Mined metal ores become pots, pans, building components, and jeweller ...
... physically before it is considered safe. Rocks and minerals form the foundation of the non-living environment. For example, magnesium carbonate from the Rocky Mountains becomes a main component of sidewalks in our bustling cities. Mined metal ores become pots, pans, building components, and jeweller ...
Metallic and nonmetallic double perovskites: A case study of A $ _2
... A further important issue is the monoclinic distortion induced by Ca. This will lift the degeneracy of the t2g levels on the Re site, making an insulating state easier to form in ...
... A further important issue is the monoclinic distortion induced by Ca. This will lift the degeneracy of the t2g levels on the Re site, making an insulating state easier to form in ...
Unit 2 – Quantities Review
... 7. Convert a mass of 2.5 g of table salt (sodium chloride) to an amount in moles. 8. Convert a mass of 1.0 kg of glucose, C6H12O6(s), to an amount in moles. 9. What is the amount in moles of 25.0 g of oxygen gas? Converting from moles to mass (g) Take the number of moles and multiply by the molar ...
... 7. Convert a mass of 2.5 g of table salt (sodium chloride) to an amount in moles. 8. Convert a mass of 1.0 kg of glucose, C6H12O6(s), to an amount in moles. 9. What is the amount in moles of 25.0 g of oxygen gas? Converting from moles to mass (g) Take the number of moles and multiply by the molar ...
Webquest and Project Guidelines
... 5. Instead of orbits, how do we refer to the special areas where electrons can be found? 6. So, what is always necessary for an electron to jumps up an energy level? 7. So, what is always released when an electron drops down an energy level? 8. What is the particle of electromagnetic energy that is ...
... 5. Instead of orbits, how do we refer to the special areas where electrons can be found? 6. So, what is always necessary for an electron to jumps up an energy level? 7. So, what is always released when an electron drops down an energy level? 8. What is the particle of electromagnetic energy that is ...
Web Quest and Poster Project Guidelines
... 5. Instead of orbits, how do we refer to the special areas where electrons can be found? 6. So, what is always necessary for an electron to jumps up an energy level? 7. So, what is always released when an electron drops down an energy level? 8. What is the particle of electromagnetic energy that is ...
... 5. Instead of orbits, how do we refer to the special areas where electrons can be found? 6. So, what is always necessary for an electron to jumps up an energy level? 7. So, what is always released when an electron drops down an energy level? 8. What is the particle of electromagnetic energy that is ...
physical setting chemistry
... Acid rain lowers the pH in ponds and lakes and over time can cause the death of some aquatic life. Acid rain is caused in large part by the burning of fossil fuels in power plants and by gasoline-powered vehicles. The acids commonly associated with acid rain are sulfurous acid, sulfuric acid, and ni ...
... Acid rain lowers the pH in ponds and lakes and over time can cause the death of some aquatic life. Acid rain is caused in large part by the burning of fossil fuels in power plants and by gasoline-powered vehicles. The acids commonly associated with acid rain are sulfurous acid, sulfuric acid, and ni ...