ض ( ا ء ا ط ك ا رر 108 1) -
... B Te C Ta D W The ionization energy is the energy needed to remove an electron from a neutral atom. A True B False The electron configuration for calcium is 1s22s22p63s23p64s2 ...
... B Te C Ta D W The ionization energy is the energy needed to remove an electron from a neutral atom. A True B False The electron configuration for calcium is 1s22s22p63s23p64s2 ...
Atoms and bonds in molecules and chemical
... of the event from a set of true propositions involving at least a scientific law or principle. The unification approach intends to derive the occurrence of the event using a theory that unifies many phenomena or the theory that unifies the phenomena better than any other. In the causal model the exp ...
... of the event from a set of true propositions involving at least a scientific law or principle. The unification approach intends to derive the occurrence of the event using a theory that unifies many phenomena or the theory that unifies the phenomena better than any other. In the causal model the exp ...
Thomson`s Model of the Atom
... Dalton’s Atomic Theory Evidence for Atoms John Dalton studied the behavior of gases in air. Based on the way gases exert pressure, Dalton correctly concluded that a gas consists of individual particles. Dalton measured masses of elements that combine when compounds form. The ratio of the masses of t ...
... Dalton’s Atomic Theory Evidence for Atoms John Dalton studied the behavior of gases in air. Based on the way gases exert pressure, Dalton correctly concluded that a gas consists of individual particles. Dalton measured masses of elements that combine when compounds form. The ratio of the masses of t ...
File - Mr Weng`s IB Chemistry
... • The mole is a fixed number of particles and refers to the amount, n, of substance. • Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/molecular mass (Mr). • Molar mass (M) has the units g mol-1. • The empirical formula and ...
... • The mole is a fixed number of particles and refers to the amount, n, of substance. • Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/molecular mass (Mr). • Molar mass (M) has the units g mol-1. • The empirical formula and ...
Core_Class_Science_Chemistry_for_the_web 838.3 KB
... Electrons are negatively charged particles (E-) located on the outside of the nucleus. Electrons constantly move around the nucleus in energy levels. Today’s Objectives: Diagram the particles that make up an atom. Compare covalent and ionic One: bonds. Answer: ...
... Electrons are negatively charged particles (E-) located on the outside of the nucleus. Electrons constantly move around the nucleus in energy levels. Today’s Objectives: Diagram the particles that make up an atom. Compare covalent and ionic One: bonds. Answer: ...
spring semester review
... 74. How many grams of Ca metal could be produced by the electrolysis of molten CaBr 2 using a current of 30.0 amp for 10.0 hours? a) 22.4 g b) 448 g c) 0.0622 g d) 224 g 75. How long will it take to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 35.2 amps? a) 5.77 minu ...
... 74. How many grams of Ca metal could be produced by the electrolysis of molten CaBr 2 using a current of 30.0 amp for 10.0 hours? a) 22.4 g b) 448 g c) 0.0622 g d) 224 g 75. How long will it take to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 35.2 amps? a) 5.77 minu ...
goyal brothers prakashan
... (ii) Electrons are negatively charged, protons are positively charged and neutrons are neutral, i.e, they have no electric charge. (iii) The number of electrons and protons are equal, so that an atom on the whole is electrically neutral. (iv) Protons and neutrons are present within the nucleus. Due ...
... (ii) Electrons are negatively charged, protons are positively charged and neutrons are neutral, i.e, they have no electric charge. (iii) The number of electrons and protons are equal, so that an atom on the whole is electrically neutral. (iv) Protons and neutrons are present within the nucleus. Due ...
- skv institute
... atoms known as electronic theory of chemical bonding. According to this - atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as octet rule. 2 Define ...
... atoms known as electronic theory of chemical bonding. According to this - atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as octet rule. 2 Define ...
Practice problem chap3 1. The atomic mass of 35Cl (75.53%) and
... 4. Chemical analysis shows the composition of a compound containing carbon and hydrogen, to be 80.00% carbon and 20% hydrogen and the molar mass is 30 g. What is its molecular formula? (a) CH (b) C2H4 (c) C2H6 (d) C6H12 (e) C10H22 5. Balance the equation a) N2O5 N2O4 +O2 f) P4O10 + H2O H3PO4 h) ...
... 4. Chemical analysis shows the composition of a compound containing carbon and hydrogen, to be 80.00% carbon and 20% hydrogen and the molar mass is 30 g. What is its molecular formula? (a) CH (b) C2H4 (c) C2H6 (d) C6H12 (e) C10H22 5. Balance the equation a) N2O5 N2O4 +O2 f) P4O10 + H2O H3PO4 h) ...
radiometric dating - Tulane University
... ppm, and thus start out with a relatively high 87Rb/86Sr ratio. Over time, this results in crustal rocks having a much higher 87Sr/86Sr ratio than mantle rocks. Thus if the mantle has a 87Sr/86Sr of say 0.7025, melting of the mantle would produce a magma with a 87Sr/86Sr ratio of 0.7025, and all roc ...
... ppm, and thus start out with a relatively high 87Rb/86Sr ratio. Over time, this results in crustal rocks having a much higher 87Sr/86Sr ratio than mantle rocks. Thus if the mantle has a 87Sr/86Sr of say 0.7025, melting of the mantle would produce a magma with a 87Sr/86Sr ratio of 0.7025, and all roc ...
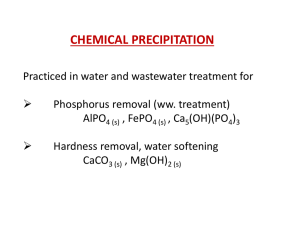
Phosphorus Removal from Wastewater by Chemical Precipitation
... However, these reactions are simple and must be considered in the light of many competing reactions. ...
... However, these reactions are simple and must be considered in the light of many competing reactions. ...
6.1 Moles and Molar Masses
... Empirical formulas can be calculated from lab data, allowing us to identify unknown compounds: STEP 1: Assume mass percentages represent masses, in g: STEP 2: Divide each element's mass by their respective molar masses, turning them into moles. STEP 3: Divide all moles by the lowest number of moles ...
... Empirical formulas can be calculated from lab data, allowing us to identify unknown compounds: STEP 1: Assume mass percentages represent masses, in g: STEP 2: Divide each element's mass by their respective molar masses, turning them into moles. STEP 3: Divide all moles by the lowest number of moles ...
Exam 961-1st Name___________________________________
... 9) Which element would have physical and chemical properties similar to chlorine? A) Ar B) Br C) P D) S ...
... 9) Which element would have physical and chemical properties similar to chlorine? A) Ar B) Br C) P D) S ...
Document
... simple calorimeters are used to measure heat changes associated with heating, cooling, phase changes, solution formation, and chemical reactions that occur in aqueous solution ...
... simple calorimeters are used to measure heat changes associated with heating, cooling, phase changes, solution formation, and chemical reactions that occur in aqueous solution ...
Ch 13 kinetics
... Provides a detailed picture of how a reaction occurs. Elementary step: Any process that occurs ____________________________________________________________________ Makes either ____________________________________________________________________________ the rate law for an __________________________ ...
... Provides a detailed picture of how a reaction occurs. Elementary step: Any process that occurs ____________________________________________________________________ Makes either ____________________________________________________________________________ the rate law for an __________________________ ...
A Classification of AP Chemistry Reactions
... Dichromate is found in redox reactions. It is a very good oxidizing agent, and is always used in acidic solution, where it forms Cr3+: - A solution of potassium iodide is added to an acidified solution of potassium dichromate. H+ + Cr2O72- + I- Cr3+ + I2 + H2O Hydrogen Peroxide Hydrogen peroxide, ...
... Dichromate is found in redox reactions. It is a very good oxidizing agent, and is always used in acidic solution, where it forms Cr3+: - A solution of potassium iodide is added to an acidified solution of potassium dichromate. H+ + Cr2O72- + I- Cr3+ + I2 + H2O Hydrogen Peroxide Hydrogen peroxide, ...
1996 Free Response Answers
... atmosphere pressure with the pure gas indicated. (a) Which balloon contains the greatest mass of gas? Explain. (b) Compare the average kinetic energies of the gas molecules in the balloons. Explain. (c) Which balloon contains the gas that would be expected to deviate most from the behavior of an ide ...
... atmosphere pressure with the pure gas indicated. (a) Which balloon contains the greatest mass of gas? Explain. (b) Compare the average kinetic energies of the gas molecules in the balloons. Explain. (c) Which balloon contains the gas that would be expected to deviate most from the behavior of an ide ...
Energetics Past Paper Questions
... The lattice enthalpy of an ionic compound can be calculated using a Born-Haber cycle. Using lithium fluoride as the example, construct a Born-Haber cycle, labelling the cycle with the formulas and state symbols of the species present at each stage. (6) Two values of the lattice enthalpies for each o ...
... The lattice enthalpy of an ionic compound can be calculated using a Born-Haber cycle. Using lithium fluoride as the example, construct a Born-Haber cycle, labelling the cycle with the formulas and state symbols of the species present at each stage. (6) Two values of the lattice enthalpies for each o ...
Energy Practice
... Q = Energy transferred to another substance as heat: Change in kinetic energy: (usually J or kJ) m = mass of substance to be considered (usually g or kg) c = the specific heat of the substance (the amount of energy required or released when one kilogram of the substance changes in temperature by 1°C ...
... Q = Energy transferred to another substance as heat: Change in kinetic energy: (usually J or kJ) m = mass of substance to be considered (usually g or kg) c = the specific heat of the substance (the amount of energy required or released when one kilogram of the substance changes in temperature by 1°C ...
The Oxidation States of Tin
... reason that this yield was low is mainly attributed to the iodine-zinc portion of this reaction. This part of the experiment required that the zinc and iodine be combined in a flask with water and allowed to sit in an ice bath for an extended amount of time. This reaction was performed five times w ...
... reason that this yield was low is mainly attributed to the iodine-zinc portion of this reaction. This part of the experiment required that the zinc and iodine be combined in a flask with water and allowed to sit in an ice bath for an extended amount of time. This reaction was performed five times w ...
Introduction to Atomic Structure - New Jersey Center for Teaching
... descending, he was able to determine the charge per drop. The more energy needed to prevent the drop from falling, the smaller the charge of the drop. ...
... descending, he was able to determine the charge per drop. The more energy needed to prevent the drop from falling, the smaller the charge of the drop. ...