Net ionic equation
... Oxidation and reduction Mg(s) +2H+(aq) Mg2+(aq) + H2(g) • In the above rxn, Mg(s) loses e-, H+ gains e• Oxidized: atom, molecule, or ion becomes more positively charged. • Reduced: atom, molecule, or ion becomes less positively charged. ...
... Oxidation and reduction Mg(s) +2H+(aq) Mg2+(aq) + H2(g) • In the above rxn, Mg(s) loses e-, H+ gains e• Oxidized: atom, molecule, or ion becomes more positively charged. • Reduced: atom, molecule, or ion becomes less positively charged. ...
Chemistry of the Non
... We divide the periodic table into metals, nonmetals and metalloids. Nonmetals occupy the upper right portion of the periodic table. • H is a special case. Electronegativity is important when determining whether an element is a metal. Nonmetals tend to have higher electronegativities than metals. • T ...
... We divide the periodic table into metals, nonmetals and metalloids. Nonmetals occupy the upper right portion of the periodic table. • H is a special case. Electronegativity is important when determining whether an element is a metal. Nonmetals tend to have higher electronegativities than metals. • T ...
class notes 4
... 4.5 Types of aqueous Solutions and Solubility Electrolyte and Nonelectrolyte Solutions Electrolyte: A substance that produces ions in a water solution and therefore conducts a current of electricity. Strong Electrolyte: A substance that dissociates completely in water solution to produce a lot of i ...
... 4.5 Types of aqueous Solutions and Solubility Electrolyte and Nonelectrolyte Solutions Electrolyte: A substance that produces ions in a water solution and therefore conducts a current of electricity. Strong Electrolyte: A substance that dissociates completely in water solution to produce a lot of i ...
Chapter 6. Therrnochemistry
... Truly balanced reactions include the energy changes that accompany chemical reactions. This chapter describes these energy changes, techniques used to measure them experimentally and methods used to predict them quantitatively. ...
... Truly balanced reactions include the energy changes that accompany chemical reactions. This chapter describes these energy changes, techniques used to measure them experimentally and methods used to predict them quantitatively. ...
Test - Angelfire
... be a hand-held device designed primarily for mathematical computations involving logarithmic and trigonometric functions and may also include graphing functions. Computers, calculators with a QWERTY keyboard, and electronic writing pads will not be allowed. Students must not bring any external suppo ...
... be a hand-held device designed primarily for mathematical computations involving logarithmic and trigonometric functions and may also include graphing functions. Computers, calculators with a QWERTY keyboard, and electronic writing pads will not be allowed. Students must not bring any external suppo ...
the scale of the electron
... in the bottom or outer layer of the pyramidal scale structure, the black circles represent free electrons. When these free electrons have jumped over to another atom, the remaining ion has a positive charge. Each white circle indicates a space where an additional free electron is required to create ...
... in the bottom or outer layer of the pyramidal scale structure, the black circles represent free electrons. When these free electrons have jumped over to another atom, the remaining ion has a positive charge. Each white circle indicates a space where an additional free electron is required to create ...
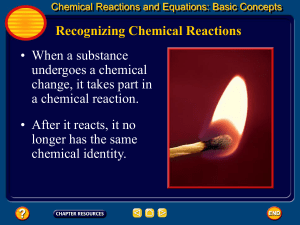
Section 4.9 Oxidation–Reduction Reactions
... If the actual yield for the previous problem was 10.5 g, calculate the percent yield. The theoretical yield that we calculated was 13.6 g. If the actual yield is 3.16 g then percent yield is ...
... If the actual yield for the previous problem was 10.5 g, calculate the percent yield. The theoretical yield that we calculated was 13.6 g. If the actual yield is 3.16 g then percent yield is ...
Chemical Equations - Salem Community Schools
... Balancing an Equation Is the equation balanced now? Two sodium atoms are on each side. How many oxygen atoms are on each side? You should be able to find four on each side. How about hydrogen atoms? Now two are on each side. Because one carbon atom is still on each side, the entire equation is balan ...
... Balancing an Equation Is the equation balanced now? Two sodium atoms are on each side. How many oxygen atoms are on each side? You should be able to find four on each side. How about hydrogen atoms? Now two are on each side. Because one carbon atom is still on each side, the entire equation is balan ...
answers to part a of the canadian chemistry
... The people involved in preparing the CCC very much appreciate all the comments and feedback that we get from teachers. We have tried to incorporate some of these comments in with the solutions. We have also tried to indicate how students did in particular questions, although, unfortunately, we have ...
... The people involved in preparing the CCC very much appreciate all the comments and feedback that we get from teachers. We have tried to incorporate some of these comments in with the solutions. We have also tried to indicate how students did in particular questions, although, unfortunately, we have ...
KS4-Rates - Free Exam Papers
... The reactions that cause the food to go off will be slower because there will be fewer and “softer” collisions between molecules at a reduced temperature. ...
... The reactions that cause the food to go off will be slower because there will be fewer and “softer” collisions between molecules at a reduced temperature. ...
Chemistry
... mass, family designation, period number, classification of element (metal, nonmetal, semimetal, or metalloid), and the state of the element at room temperature. Identify regions of the periodic table including alkali metals, alkaline earth metals, transition metals, halogens, noble gases, lanthanide ...
... mass, family designation, period number, classification of element (metal, nonmetal, semimetal, or metalloid), and the state of the element at room temperature. Identify regions of the periodic table including alkali metals, alkaline earth metals, transition metals, halogens, noble gases, lanthanide ...
SUPPLEMENTAL PROBLEMS FOR CHEM 110
... 10. Which series of quantum numbers describes the orbital in which the highest energy electron in potassium resides in the ground state? ...
... 10. Which series of quantum numbers describes the orbital in which the highest energy electron in potassium resides in the ground state? ...
Mass and Stoichiometry
... Example: Lead ore, Lead sulfide (with the common name “galena”), was “calcined” by early metallurgists to form a lead oxide used to purify silver from other metals. Calcined means to burn in the presence of oxygen. At low temperatures the yellow lead oxide PbO, litharge, is formed. At higher tempera ...
... Example: Lead ore, Lead sulfide (with the common name “galena”), was “calcined” by early metallurgists to form a lead oxide used to purify silver from other metals. Calcined means to burn in the presence of oxygen. At low temperatures the yellow lead oxide PbO, litharge, is formed. At higher tempera ...
a) How many moles of water are created when 108 moles of oxygen
... b) How many litres of carbon dioxide gas will be created with 525 L of oxygen gas react completely at STP? Hint this can be done with a single conversion! ...
... b) How many litres of carbon dioxide gas will be created with 525 L of oxygen gas react completely at STP? Hint this can be done with a single conversion! ...
1 Intro / Review : Chemical Kinetics
... Enduring understanding 4.A: Reaction rates that depend on temperature and other environmental factors are determined by measuring changes in concentrations of reactants or products over time. Essential knowledge 4.A.2: The rate law shows how the rate depends on reactant concentrations. Essential kno ...
... Enduring understanding 4.A: Reaction rates that depend on temperature and other environmental factors are determined by measuring changes in concentrations of reactants or products over time. Essential knowledge 4.A.2: The rate law shows how the rate depends on reactant concentrations. Essential kno ...
CHAPTER-8 NCERT SOLUTIONS
... Adding the two half reactions, we have the net balanced redox reaction as: (b)Following the steps as in part (a), we have the oxidation half reaction as: And the reduction half reaction as: Multiplying the oxidation half reaction by 5 and the reduction half reaction by 2, and then by adding them, we ...
... Adding the two half reactions, we have the net balanced redox reaction as: (b)Following the steps as in part (a), we have the oxidation half reaction as: And the reduction half reaction as: Multiplying the oxidation half reaction by 5 and the reduction half reaction by 2, and then by adding them, we ...
Particles
... Binary Compounds and Binary Ions are made of two elements (not necessarily two atoms). ...
... Binary Compounds and Binary Ions are made of two elements (not necessarily two atoms). ...
Open questions (66 points total
... (NOTE There are 2 NMR spectra with this problem. Below the 1H spectrum, the integrals (= areas) of the signals are given as numbers ratios). From the IR spectrum of an unknown substance X with M = 102, we know X to be an ester. 6p 1 Calculate the molecular formula of substance X. Give all possible ...
... (NOTE There are 2 NMR spectra with this problem. Below the 1H spectrum, the integrals (= areas) of the signals are given as numbers ratios). From the IR spectrum of an unknown substance X with M = 102, we know X to be an ester. 6p 1 Calculate the molecular formula of substance X. Give all possible ...
PREPARATORY PROBLEMS (Theoretical)
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
Spring 2014
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
PREPARATORY PROBLEMS (Theoretical)
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
1 Unit 11-12: Equilibrium and Acid/Bases Notes Colligative
... 8. Which change may occur when a catalyst is added to a reaction system? a. activation energy for the reaction decreases b. the heat of the reaction decreases c. the potential energy of the reactants increases d. the potential energy of the products decreases ...
... 8. Which change may occur when a catalyst is added to a reaction system? a. activation energy for the reaction decreases b. the heat of the reaction decreases c. the potential energy of the reactants increases d. the potential energy of the products decreases ...
1. Bromine exists naturally as a mixture of bromine
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.60 1013 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each r ...
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.60 1013 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each r ...