Atoms: The Building Blocks of Matter
... Democritus’s idea into a scientific theory that could be tested by experiment. But not all aspects of Dalton’s atomic theory have proven to be correct. For example, today we know that atoms are divisible into even smaller particles (although the law of conservation of mass still holds true for chemi ...
... Democritus’s idea into a scientific theory that could be tested by experiment. But not all aspects of Dalton’s atomic theory have proven to be correct. For example, today we know that atoms are divisible into even smaller particles (although the law of conservation of mass still holds true for chemi ...
Aqueous chemistry is a very important component to laboratory
... In precipitation reactions, two soluble ionic compounds come together to form a solid, insoluble product, called the precipitate. Precipitates form for the same reason that some ionic compounds do not dissolve in water: the electrostatic attraction between the ions outweighs the tendency of the ions ...
... In precipitation reactions, two soluble ionic compounds come together to form a solid, insoluble product, called the precipitate. Precipitates form for the same reason that some ionic compounds do not dissolve in water: the electrostatic attraction between the ions outweighs the tendency of the ions ...
Reaction Analysis and PAT Tools
... iC IR™ software was designed to take infrared data and convert it into useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of th ...
... iC IR™ software was designed to take infrared data and convert it into useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of th ...
The Advanced Placement Examination in Chemistry Part I – Multiple
... (d) Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO2 and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. ...
... (d) Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO2 and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. ...
PPT - mvhs-fuhsd.org
... that occur in the course of a reaction.Each of these steps are called as elementary steps. An elementary step may produce an intermediate, a product that is consumed in a later elementary step and therefore does not appear in the overall stoichiometry of the reaction. If a mechanism has several elem ...
... that occur in the course of a reaction.Each of these steps are called as elementary steps. An elementary step may produce an intermediate, a product that is consumed in a later elementary step and therefore does not appear in the overall stoichiometry of the reaction. If a mechanism has several elem ...
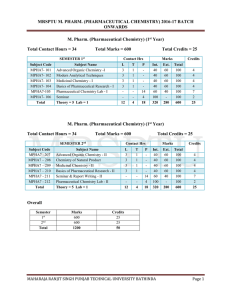
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
Structure of the Nuclear Atom
... paraffin wax, which has a high concentration of hydrogen and is therefore rich in protons. Some of the neutrons collided with protons in the wax and knocked them out. Chadwick could then detect these protons and measure their energy. Using his knowledge of energy and momentum, he was able to work ou ...
... paraffin wax, which has a high concentration of hydrogen and is therefore rich in protons. Some of the neutrons collided with protons in the wax and knocked them out. Chadwick could then detect these protons and measure their energy. Using his knowledge of energy and momentum, he was able to work ou ...
Unit 8: Reactions
... NO3’s in the reactants and three NO3’s in the products. Again, it is (2 x 3 = 6), so we’ll need six nitrate ions on either side of the arrow. ix. Place a coefficient of for the reactant with nitrate, and a coefficient of ...
... NO3’s in the reactants and three NO3’s in the products. Again, it is (2 x 3 = 6), so we’ll need six nitrate ions on either side of the arrow. ix. Place a coefficient of for the reactant with nitrate, and a coefficient of ...
- University Of Nigeria Nsukka
... In principle, electronic ,pictures a s illustrated in (i), (ii) and (iii) above can be drawn for any molecule and the bonding electrons assigned arbitrarily to the more electronegative element. However, deciding oxidation numbers by this approach is quite laborious. In practice, oxidation numbers ar ...
... In principle, electronic ,pictures a s illustrated in (i), (ii) and (iii) above can be drawn for any molecule and the bonding electrons assigned arbitrarily to the more electronegative element. However, deciding oxidation numbers by this approach is quite laborious. In practice, oxidation numbers ar ...
EVANS GROUP RESEARCH PROJECT DESCRIPTIONS
... Divalent Complexes and Sterically Induced Reduction Reactivity of Trivalent Complexes" Journal of Organometallic Chemistry 2002, 647, 2-11. "Formal Three Electron Reduction by an f element Complex: Formation of [(C5Me5)(C8H8)U]2(C8H8) from Cycloctatetraene and (C5Me5)3U" Angewandte Chemie, Internati ...
... Divalent Complexes and Sterically Induced Reduction Reactivity of Trivalent Complexes" Journal of Organometallic Chemistry 2002, 647, 2-11. "Formal Three Electron Reduction by an f element Complex: Formation of [(C5Me5)(C8H8)U]2(C8H8) from Cycloctatetraene and (C5Me5)3U" Angewandte Chemie, Internati ...
James W. Whittaker - Oxygen reactions of the copper oxidases
... product of the reaction. The reactivity of Cu towards O2 depends on the nuclearity, or number of metal ions, which determines the total number of electrons a complex can deliver, as well as the redox potential of the metal complex. The importance of nuclearity is illustrated by the binuclear Cu(I)–C ...
... product of the reaction. The reactivity of Cu towards O2 depends on the nuclearity, or number of metal ions, which determines the total number of electrons a complex can deliver, as well as the redox potential of the metal complex. The importance of nuclearity is illustrated by the binuclear Cu(I)–C ...
Coherent/incoherent light production from wide
... particle model and the wave model. A particle is an object that can be represented as a point in space which transfers matter and energy as it moves through a field. A wave, in contrast to a particle, is a disturbance that transfers energy from one place to another without any net transfer of matter ...
... particle model and the wave model. A particle is an object that can be represented as a point in space which transfers matter and energy as it moves through a field. A wave, in contrast to a particle, is a disturbance that transfers energy from one place to another without any net transfer of matter ...
hty utI! rn h 1m 0 nt - Northside Middle School
... You might think that because atoms are so small there would be no way to actually see them. However, an instrument called the scanning tunneling microscope allows individual atoms to be seen. Do the problem-solving LAB on page 96 to analyze scanning tunneling microscope images and gain a better unde ...
... You might think that because atoms are so small there would be no way to actually see them. However, an instrument called the scanning tunneling microscope allows individual atoms to be seen. Do the problem-solving LAB on page 96 to analyze scanning tunneling microscope images and gain a better unde ...
4.1 Studying Atoms
... consisted of extremely small particles that could not be divided. 2. Aristotle a. Stated that all substances were made of only four elements—earth, air, fire, and water. ...
... consisted of extremely small particles that could not be divided. 2. Aristotle a. Stated that all substances were made of only four elements—earth, air, fire, and water. ...
Chemical reaction model:
... The process of natural oxidative degradation takes years and it is not possible to wait that long for data to be available when testing new materials. Hence, the oxidation of polymer is frequently carried at elevated temperature and pressure of oxygen. Elevation in temperature and pressure leads to ...
... The process of natural oxidative degradation takes years and it is not possible to wait that long for data to be available when testing new materials. Hence, the oxidation of polymer is frequently carried at elevated temperature and pressure of oxygen. Elevation in temperature and pressure leads to ...
Elemental Analysis
... The detection of individual elements in a mixture with other accompanying elements is a rather difficult problem, because all of them can interact with the same reagents with a similar outward effect. Using specific reagents and reactions, makes it possible to detect some elements in mixtures with a ...
... The detection of individual elements in a mixture with other accompanying elements is a rather difficult problem, because all of them can interact with the same reagents with a similar outward effect. Using specific reagents and reactions, makes it possible to detect some elements in mixtures with a ...
uplift luna ap chemistry
... CnH2n+1OH; Do not be fooled—this looks like a hydroxide ion, but is not! It does not make this hydrocarbon an alkaline or basic compound. Do not name these as a hydroxide! C2H6 is ethane while C2H5OH is ethanol. NAMING BINARY IONIC COMPOUNDS How do I know it is ionic? The chemical formula will begin ...
... CnH2n+1OH; Do not be fooled—this looks like a hydroxide ion, but is not! It does not make this hydrocarbon an alkaline or basic compound. Do not name these as a hydroxide! C2H6 is ethane while C2H5OH is ethanol. NAMING BINARY IONIC COMPOUNDS How do I know it is ionic? The chemical formula will begin ...
Topic 1: Quantitative chemistry (12
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
Full answers
... As the reaction is endothermic, the energy of the products is higher than that of the reactants. Would you expect the forward or the reverse reaction to be faster? Why? The backward reaction would be faster as it has a lower activation energy. This is a consequence of the reaction being endothermic. ...
... As the reaction is endothermic, the energy of the products is higher than that of the reactants. Would you expect the forward or the reverse reaction to be faster? Why? The backward reaction would be faster as it has a lower activation energy. This is a consequence of the reaction being endothermic. ...
Topic 1: Quantitative chemistry (12
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
17 ADSORPTION AND CATALYSIS S MODULE - 5
... Adsorption Isotherm : One of the drawbacks of the Freundlich adsorption isotherm is that it fails at high pressure of the gas. Langmuir derived an adsorption isotherm on theoretical considerations based on kinetic theory of gases. This is named as the Langmuir adosrption isotherm. This isotherm is b ...
... Adsorption Isotherm : One of the drawbacks of the Freundlich adsorption isotherm is that it fails at high pressure of the gas. Langmuir derived an adsorption isotherm on theoretical considerations based on kinetic theory of gases. This is named as the Langmuir adosrption isotherm. This isotherm is b ...
Chemistry
... Compounds of nitrogen: ammonia – manufacture by Haber’s process, properties – basic character, reaction with ZnSO4 and Cu2+ ion. Nitric acid – manufacture by Ostwald’s process , laboratory method – from NaNO3, properties – oxidizing properties – dilute HNO3 with Zn and Cu, concentrated HNO3 with Cu, ...
... Compounds of nitrogen: ammonia – manufacture by Haber’s process, properties – basic character, reaction with ZnSO4 and Cu2+ ion. Nitric acid – manufacture by Ostwald’s process , laboratory method – from NaNO3, properties – oxidizing properties – dilute HNO3 with Zn and Cu, concentrated HNO3 with Cu, ...