aq - Byron High School
... zero (rule 4). Letting x equal the oxidation number of S, we have 2(+1) + x = 0. Thus, S has an oxidation number of –2. (b) Because this is an elemental form of sulfur, the oxidation number of S is 0 (rule 1). (c) Because this is a binary compound, we expect chlorine to have an oxidation number of – ...
... zero (rule 4). Letting x equal the oxidation number of S, we have 2(+1) + x = 0. Thus, S has an oxidation number of –2. (b) Because this is an elemental form of sulfur, the oxidation number of S is 0 (rule 1). (c) Because this is a binary compound, we expect chlorine to have an oxidation number of – ...
Coordination and Chemistry of Stable Cu (II) Complexes in the Gas
... there is destruction of the cluster beam due to scattering, and at lower partial pressures insufficient quantities of metal are picked up. A shutter at the exit of the effusion cell is used to confirm the presence of copper in the clusters. Where a survey is performed of the relative intensities of ...
... there is destruction of the cluster beam due to scattering, and at lower partial pressures insufficient quantities of metal are picked up. A shutter at the exit of the effusion cell is used to confirm the presence of copper in the clusters. Where a survey is performed of the relative intensities of ...
REVIEW and answers
... What kind of intermolecular force is involved in Au ? Why does this force occur ? What physical properties result from this force ? Au is a metal, so metallic bonding occurs between atoms of gold. Metallic bonding is based on the existence of loosely held outer electrons which become delocalized; th ...
... What kind of intermolecular force is involved in Au ? Why does this force occur ? What physical properties result from this force ? Au is a metal, so metallic bonding occurs between atoms of gold. Metallic bonding is based on the existence of loosely held outer electrons which become delocalized; th ...
Chemistry 12 - Correspondence Studies
... The amount of energy involved in a chemical reaction can be measured using an instrument called a calorimeter. A simple laboratory calorimeter is shown on page 357 of the text. The polystyrene cup acts as an insulator to reduce heat flow to the surroundings. The chemical reaction takes place in the ...
... The amount of energy involved in a chemical reaction can be measured using an instrument called a calorimeter. A simple laboratory calorimeter is shown on page 357 of the text. The polystyrene cup acts as an insulator to reduce heat flow to the surroundings. The chemical reaction takes place in the ...
Chapter 10 - Chemical Quantities
... 21. Find the empirical formula of a compound, given that the compound is found to be 47.9% zinc (Zn) and 52.1% chlorine (Cl) by mass. (Zn = 65.4 g/mol; Cl = 35.5 g/mol) Ans: ZnCl2 22. Find the empirical formula of a compound, given that a 48.5-g sample of the compound is found to contain 1.75 g of c ...
... 21. Find the empirical formula of a compound, given that the compound is found to be 47.9% zinc (Zn) and 52.1% chlorine (Cl) by mass. (Zn = 65.4 g/mol; Cl = 35.5 g/mol) Ans: ZnCl2 22. Find the empirical formula of a compound, given that a 48.5-g sample of the compound is found to contain 1.75 g of c ...
CHAPTER 3
... Charge and Mass of the Electron Cathode rays have identical properties regardless of the element used to produce them. Therefore, it was concluded that electrons are present in atoms of all elements. Thus, cathode-ray experiments provided evidence that atoms are divisible and that one of the atom’s ...
... Charge and Mass of the Electron Cathode rays have identical properties regardless of the element used to produce them. Therefore, it was concluded that electrons are present in atoms of all elements. Thus, cathode-ray experiments provided evidence that atoms are divisible and that one of the atom’s ...
AP Chemistry Summer Assignment
... Where Q= any nonmetal and X=a different nonmetal y and z are small integers Steps in naming binary covalent compounds: 1. Write the numerical prefix for y (unless it is one then no numerical prefix is used) 2. Add the full name of the nonmetal Q 3. Write the numerical prefix of z (unless it is one t ...
... Where Q= any nonmetal and X=a different nonmetal y and z are small integers Steps in naming binary covalent compounds: 1. Write the numerical prefix for y (unless it is one then no numerical prefix is used) 2. Add the full name of the nonmetal Q 3. Write the numerical prefix of z (unless it is one t ...
The Major Classes of Chemical Reactions
... The Polar Nature of Water Of the many thousands of reactions that occur in the environment and in organisms, nearly all take place in water. Water’s remarkable power as a solvent results from two features of its molecules: the distribution of the bonding electrons and the overall shape. Recall from ...
... The Polar Nature of Water Of the many thousands of reactions that occur in the environment and in organisms, nearly all take place in water. Water’s remarkable power as a solvent results from two features of its molecules: the distribution of the bonding electrons and the overall shape. Recall from ...
Chapter 5 Chemical Equilibrium 1 State whether each of the
... (c) You should have found that Hvap is smaller at the higher temperature. Why is this so? This is because at higher temperature, the water molecules already have higher energy, so less is required to vaporize them from the liquid into the gas phase. ...
... (c) You should have found that Hvap is smaller at the higher temperature. Why is this so? This is because at higher temperature, the water molecules already have higher energy, so less is required to vaporize them from the liquid into the gas phase. ...
4.1 Studying Atoms
... • All elements are composed of atoms. • All atoms of the same element have the same mass, and atoms of different elements have different masses. • Compounds contain atoms of more than one element. • In a particular compound, atoms of different elements always combine in the same way. ...
... • All elements are composed of atoms. • All atoms of the same element have the same mass, and atoms of different elements have different masses. • Compounds contain atoms of more than one element. • In a particular compound, atoms of different elements always combine in the same way. ...
THE MOLE (pp. 159
... ***** Determine the empirical formula of a compound containing carbon, hydrogen and nitrogen given the following data: ...
... ***** Determine the empirical formula of a compound containing carbon, hydrogen and nitrogen given the following data: ...
General Chemistry: Atoms First (McMurry/Fay/Pribush)
... Chapter 2 The Structure and Stability of Atoms 2.1 Multiple Choice Questions 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the w ...
... Chapter 2 The Structure and Stability of Atoms 2.1 Multiple Choice Questions 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the w ...
Atomic Structure
... For the energy levels in an atom, which one of the following statement is incorrect? (a) There are seven principle electron energy levels (b) The second principal energy level can have four sub-energy levels and contain a Maximum of eight electrons (c) The M energy level can have a maximum of 32 ele ...
... For the energy levels in an atom, which one of the following statement is incorrect? (a) There are seven principle electron energy levels (b) The second principal energy level can have four sub-energy levels and contain a Maximum of eight electrons (c) The M energy level can have a maximum of 32 ele ...
Chapter 14 CHEMICAL KINETICS
... A nucleophile “loves nucleus” is a species with a region of negative or partial negative charge, such as: o anions (Cl‒ ion) , or o the negative region of polar molecules like HCl. Nucleophiles are electron-rich. ...
... A nucleophile “loves nucleus” is a species with a region of negative or partial negative charge, such as: o anions (Cl‒ ion) , or o the negative region of polar molecules like HCl. Nucleophiles are electron-rich. ...
Atoms and bonds in molecules and chemical explanations
... selectively, so that the transmitted light has a different spectrum from that of sunlight; but a chemist would answer that it is because ordinary glass contains ferrous ions. This example shows that several causal processes may be invoked to explain a fact. In the present case all answers are releva ...
... selectively, so that the transmitted light has a different spectrum from that of sunlight; but a chemist would answer that it is because ordinary glass contains ferrous ions. This example shows that several causal processes may be invoked to explain a fact. In the present case all answers are releva ...
MT 3 Practice
... the value that should be used for n in the Nernst equation is [A] 5. [B] 6. [C] 10. [D] 2. ...
... the value that should be used for n in the Nernst equation is [A] 5. [B] 6. [C] 10. [D] 2. ...
IB Chemistry HL Topic5 Questions 1. Which combination of ionic
... Two values of the lattice enthalpies for each of the silver halides are quoted in the Data Booklet. Discuss the bonding in silver fluoride and in silver iodide, with reference to ...
... Two values of the lattice enthalpies for each of the silver halides are quoted in the Data Booklet. Discuss the bonding in silver fluoride and in silver iodide, with reference to ...
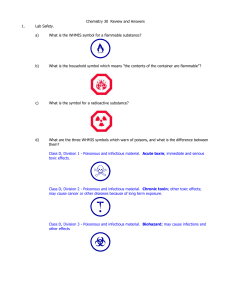
Brochure BITSAT-2011
... to score higher. However, candidates should keep in mind the fact that there is negative marking for wrong answers and any attempt to answer the questions by pure guessing of the answers is not likely to have any advantage, but may result in a reduction in the total score. The questions will be sele ...
... to score higher. However, candidates should keep in mind the fact that there is negative marking for wrong answers and any attempt to answer the questions by pure guessing of the answers is not likely to have any advantage, but may result in a reduction in the total score. The questions will be sele ...
Chapter 4 Nomenclature and Chemical Equations
... It is a convention to write the reactants on the left and the products on the right. The letters enclosed in the parenthesis tell us the states of the substances: s denotes a solid, l denotes a liquid, g denotes a gas and aq denotes an aqueous solution, i.e. a homogeneous mixture in water. Theref ...
... It is a convention to write the reactants on the left and the products on the right. The letters enclosed in the parenthesis tell us the states of the substances: s denotes a solid, l denotes a liquid, g denotes a gas and aq denotes an aqueous solution, i.e. a homogeneous mixture in water. Theref ...
+ 2 H2O(l Ca(OH)2 aq)
... H2O2 is the oxidizing agent (O.N.(O) goes from -1 to -2). Cr(OH)3 is the reducing agent (O.N.(Cr) goes from +3 to +6). b) 4 MnO4–(aq) + 3 ClO2–(aq) + 2 H2O(l) 4 MnO2(s) + 3 ClO4–(aq) + 4 OH–(aq) MnO4– is the oxidizing agent (O.N.(Mn) goes from +7 to +4). ClO2– is the reducing agent (O.N.(Cl) goes ...
... H2O2 is the oxidizing agent (O.N.(O) goes from -1 to -2). Cr(OH)3 is the reducing agent (O.N.(Cr) goes from +3 to +6). b) 4 MnO4–(aq) + 3 ClO2–(aq) + 2 H2O(l) 4 MnO2(s) + 3 ClO4–(aq) + 4 OH–(aq) MnO4– is the oxidizing agent (O.N.(Mn) goes from +7 to +4). ClO2– is the reducing agent (O.N.(Cl) goes ...
CHAPTER 9 CHEMICAL BONDING I
... The octet rule, formulated by Lewis, states that an atom other than hydrogen tends to form bonds until it is surrounded by eight valence electrons. In other words, a covalent bond forms when there are not enough electrons for each individual atom to have a complete octet. By sharing electrons in a c ...
... The octet rule, formulated by Lewis, states that an atom other than hydrogen tends to form bonds until it is surrounded by eight valence electrons. In other words, a covalent bond forms when there are not enough electrons for each individual atom to have a complete octet. By sharing electrons in a c ...
The Greek Concept of Atomos: The Indivisible Atom - Mr
... In 1803, John Dalton of England introduced the atomic idea to chemistry (and is called the Father of Modern Atomic Theory for his efforts). However, it would be false to assume that atomic ideas disappeared completely from the intellectual map for over 2000 years. For, although atomic thinkers betwe ...
... In 1803, John Dalton of England introduced the atomic idea to chemistry (and is called the Father of Modern Atomic Theory for his efforts). However, it would be false to assume that atomic ideas disappeared completely from the intellectual map for over 2000 years. For, although atomic thinkers betwe ...