Dalton`s Atomic Theory
... • All substances are made of atoms. Atoms are the smallest particles of matter. They cannot be divided into smaller particles, created, or destroyed. • All atoms of the same element are alike and have the same mass. Atoms of different elements are different and have different masses. • Atoms join to ...
... • All substances are made of atoms. Atoms are the smallest particles of matter. They cannot be divided into smaller particles, created, or destroyed. • All atoms of the same element are alike and have the same mass. Atoms of different elements are different and have different masses. • Atoms join to ...
RxnTypesPrednotesIIAP
... reactions are said to be reversible - that is, once the products are formed, they may turn back into the original reactants. The reactions that we will be considering are said to be irreversible. Two criteria must be met for a reaction to have occurred. (1) Both reactants must be soluble in water or ...
... reactions are said to be reversible - that is, once the products are formed, they may turn back into the original reactants. The reactions that we will be considering are said to be irreversible. Two criteria must be met for a reaction to have occurred. (1) Both reactants must be soluble in water or ...
Introduction to Chemistry
... stronger than women; therefore, it was logical to him that men would have more teeth than women. Thus, Aristotle concluded it was a true fact that men had more teeth than women. Apparently, it never entered his mind to actually look into the mouths of both genders and count their teeth. Had he done ...
... stronger than women; therefore, it was logical to him that men would have more teeth than women. Thus, Aristotle concluded it was a true fact that men had more teeth than women. Apparently, it never entered his mind to actually look into the mouths of both genders and count their teeth. Had he done ...
SCHLOSS RINGBERG
... the nitric oxide anion and resulting in the excitation of electrons. In order to quantify the exact amount of energy transferred to the surface, the molecules final translational and rotational energy has to be known in addition to the final vibrational energy. Time-offlight experiments on initially ...
... the nitric oxide anion and resulting in the excitation of electrons. In order to quantify the exact amount of energy transferred to the surface, the molecules final translational and rotational energy has to be known in addition to the final vibrational energy. Time-offlight experiments on initially ...
Get Solutions - Iqraa group of institutes
... here ZnO acts as an base ZnO is an amphoteric oxide but in given reaction. 25. The radius of the second Bohr orbit for hydrogen atom is : (Planck’s Const. H= 6.6262×10-34 Js; mass of electr0n=9.1091×10-31 kg; charge of electron e = 1.60210×10-19 C; permittivity of vacuum ...
... here ZnO acts as an base ZnO is an amphoteric oxide but in given reaction. 25. The radius of the second Bohr orbit for hydrogen atom is : (Planck’s Const. H= 6.6262×10-34 Js; mass of electr0n=9.1091×10-31 kg; charge of electron e = 1.60210×10-19 C; permittivity of vacuum ...
Chemistry 8.2
... Double-Displacement Reactions • In double-displacement reactions, the ions of two compounds exchange places in an aqueous solution to form two new compounds. • One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually wa ...
... Double-Displacement Reactions • In double-displacement reactions, the ions of two compounds exchange places in an aqueous solution to form two new compounds. • One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually wa ...
File
... production; HNO3: important industrial chemical, used to form nitrogen-based explosives, strong acid and a very strong oxidizing agent. ...
... production; HNO3: important industrial chemical, used to form nitrogen-based explosives, strong acid and a very strong oxidizing agent. ...
Thermochemistry
... The internal energy of a system has two components: kinetic and potential energy. Kinetic energy - the energy produced by a moving object (various types of molecular motion and the movement of electrons within molecules). Potential energy - energy available by virtue of an object’s position (determ ...
... The internal energy of a system has two components: kinetic and potential energy. Kinetic energy - the energy produced by a moving object (various types of molecular motion and the movement of electrons within molecules). Potential energy - energy available by virtue of an object’s position (determ ...
Document
... What can geochemistry do in 2004? • The earth is made of 90 or so chemical elements, about 30 w/isotopic variations • Chemical/isotopic characteristics can be tied to geological processes - mantle isotopic chemistry is a tracer • We can tell where a particular piece of mantle has been in the past a ...
... What can geochemistry do in 2004? • The earth is made of 90 or so chemical elements, about 30 w/isotopic variations • Chemical/isotopic characteristics can be tied to geological processes - mantle isotopic chemistry is a tracer • We can tell where a particular piece of mantle has been in the past a ...
Chemical Equations and Reactions
... products. Remember what you learned in Chapter 7 about symbols and formulas. Knowledge of the common oxidation states of the elements and of methods of writing formulas will enable you to supply formulas for reactants and products if they are not available. Recall that the elements listed in Table 8 ...
... products. Remember what you learned in Chapter 7 about symbols and formulas. Knowledge of the common oxidation states of the elements and of methods of writing formulas will enable you to supply formulas for reactants and products if they are not available. Recall that the elements listed in Table 8 ...
Energy
... The negative sign in the above equation occurs because we are measuring the value of q for the surroundings, and qsys = - qsur. If we know the energy of combustion for a compound, in units of kJ/g, then we can say q = m Ucom m = mass of compound burned Ucom = energy of combustion (in kJ/g) Note th ...
... The negative sign in the above equation occurs because we are measuring the value of q for the surroundings, and qsys = - qsur. If we know the energy of combustion for a compound, in units of kJ/g, then we can say q = m Ucom m = mass of compound burned Ucom = energy of combustion (in kJ/g) Note th ...
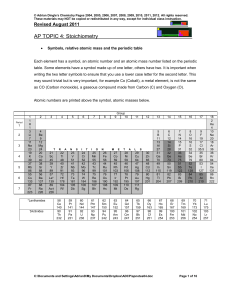
Topic #4 Notes
... Average relative atomic masses are the mass numbers recorded on the periodic table. The relative masses of atoms shown on the periodic table can be used to determine the relative masses of molecules and ions by simple summation. Relative Molecular Mass (RMM) or Molar Mass - Found by adding all of th ...
... Average relative atomic masses are the mass numbers recorded on the periodic table. The relative masses of atoms shown on the periodic table can be used to determine the relative masses of molecules and ions by simple summation. Relative Molecular Mass (RMM) or Molar Mass - Found by adding all of th ...
PRACTICE FINAL EXAM CHEMISTRY 152 This
... This practice final represents the format and general types of questions that you should expect to see on the final exam. Do not limit your studying to completing this final – you can also go back to old tests and “redo” them, redo Mastering Chemistry assignments for practice, study from old quizzes ...
... This practice final represents the format and general types of questions that you should expect to see on the final exam. Do not limit your studying to completing this final – you can also go back to old tests and “redo” them, redo Mastering Chemistry assignments for practice, study from old quizzes ...
History of the Atom
... Based on his results, Rutherford concluded that all the positive charge of an atom is concentrated in a small central area. He called this area the nucleus. Rutherford later discovered that the nucleus contains positively charged particles. He named the positive particles protons. Rutherford also pr ...
... Based on his results, Rutherford concluded that all the positive charge of an atom is concentrated in a small central area. He called this area the nucleus. Rutherford later discovered that the nucleus contains positively charged particles. He named the positive particles protons. Rutherford also pr ...
L22 - Supplementary Student Notes Package
... For each of the following questions, write a balanced chemical equation and then use the mole ratio to answer the question. 1. A student mixes together a solution of silver nitrate with a solution of sodium chromate and a precipitate forms. What amount of precipitate will form if the student has rea ...
... For each of the following questions, write a balanced chemical equation and then use the mole ratio to answer the question. 1. A student mixes together a solution of silver nitrate with a solution of sodium chromate and a precipitate forms. What amount of precipitate will form if the student has rea ...
An enquiry into theoretical bioinorganic chemistry: How heuristic is
... DFT—because of the feasibility of such calculations rather than because of their reliability. In principle, there exists an exact energy density functional that allows us to calculate the potential energy surface Eel,0 of the electronic ground state from the electronic density alone owing to the fir ...
... DFT—because of the feasibility of such calculations rather than because of their reliability. In principle, there exists an exact energy density functional that allows us to calculate the potential energy surface Eel,0 of the electronic ground state from the electronic density alone owing to the fir ...
I - Holland Public Schools
... *When you look at the equations above on paper, there is no way to tell which one is faster It can only be determined by experiment * reaction rate is affected by two factors: - collision effectiveness – an effective collision is one in which product is formed - collision frequency- a measure of how ...
... *When you look at the equations above on paper, there is no way to tell which one is faster It can only be determined by experiment * reaction rate is affected by two factors: - collision effectiveness – an effective collision is one in which product is formed - collision frequency- a measure of how ...
Ch. 8.3 – Bonding Theories
... Pi Bonds In a pi bond (symbolized by the Greek letter ), the bonding electrons are most likely to be found in sausage-shaped regions above and below the bond axis of the bonded atoms. ...
... Pi Bonds In a pi bond (symbolized by the Greek letter ), the bonding electrons are most likely to be found in sausage-shaped regions above and below the bond axis of the bonded atoms. ...
Slide 1
... • Thomson found that the mass to charge ratio of the electron was -5.686x10-12 kg/C • Millikan found the charge on the electron was -1.62 x 10-19 C. (C is the abbreviation for the coulomb, a unit of charge.) • Therefore the mass of the electron can be calculated: mass = charge x mass/charge • mass ...
... • Thomson found that the mass to charge ratio of the electron was -5.686x10-12 kg/C • Millikan found the charge on the electron was -1.62 x 10-19 C. (C is the abbreviation for the coulomb, a unit of charge.) • Therefore the mass of the electron can be calculated: mass = charge x mass/charge • mass ...
Heat
... 1) Since C = q/T, the units for C are (energy)/(temperature). The derived MKS units for C are J/K, though it is often given in J/C. 2) Note that the numerical value for C when expressed in J/K or J/C is identical. That is because we are using the change in temperature. Since the size of a degree ...
... 1) Since C = q/T, the units for C are (energy)/(temperature). The derived MKS units for C are J/K, though it is often given in J/C. 2) Note that the numerical value for C when expressed in J/K or J/C is identical. That is because we are using the change in temperature. Since the size of a degree ...