D01 Atomic Models.notebook
... Einstein predicted that an excited electron could be "encouraged" to drop to its lower energy state. This is accomplished by directing at the excited atom photons that have the same frequency as the photons that will be emitted during the electron transition. These incident photons are not ab ...
... Einstein predicted that an excited electron could be "encouraged" to drop to its lower energy state. This is accomplished by directing at the excited atom photons that have the same frequency as the photons that will be emitted during the electron transition. These incident photons are not ab ...
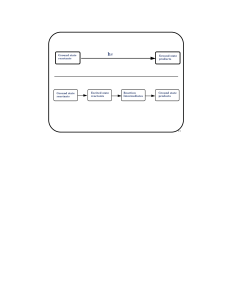
Ground state reactants Ground state products Ground state
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
Minerals - UNLV Geoscience
... • Central region called the nucleus – Consists of protons (positive charges) and neutrons (neutral charges) ...
... • Central region called the nucleus – Consists of protons (positive charges) and neutrons (neutral charges) ...
Energetics - chemistryatdulwich
... A chemical reaction results in a change in enthalpy because during any chemical reaction old bonds are broken and new bonds of different strengths are being made. When we change the bonds within a system, we are changing the way atoms and electrons interact with each other, we change the way in whic ...
... A chemical reaction results in a change in enthalpy because during any chemical reaction old bonds are broken and new bonds of different strengths are being made. When we change the bonds within a system, we are changing the way atoms and electrons interact with each other, we change the way in whic ...
Document
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
1 of 52
... The following questions represent the baseline knowledge you will need to succeed in AP Chemistry. The goal of this assignment is twofold. First, it will tell you what you are up against in the upcoming school year. Second, this assignment covers curriculum that is basic and will allow us the time t ...
... The following questions represent the baseline knowledge you will need to succeed in AP Chemistry. The goal of this assignment is twofold. First, it will tell you what you are up against in the upcoming school year. Second, this assignment covers curriculum that is basic and will allow us the time t ...
C5 Chemicals of the Natural Environment SOW
... Difficulty in visualising the forces within and between molecules may leave students still confused as to why molecules do not break up at their boiling points. Ionic compounds It could confuse students that many compounds are ionic but are not found in seawater, are insoluble in water and do not ha ...
... Difficulty in visualising the forces within and between molecules may leave students still confused as to why molecules do not break up at their boiling points. Ionic compounds It could confuse students that many compounds are ionic but are not found in seawater, are insoluble in water and do not ha ...
Document
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
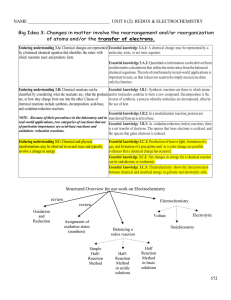
Unit - II Electrochemistry
... Lecturer-II /IV Corrosion-Introduction, types Mechanism of dry and wet corrosion “Corrosion is defined as the gradual destruction of metals or alloys by the chemical or electrochemical reaction with its environment.” Causes of corrosion occurs Most of the metals (except noble metals) naturally exis ...
... Lecturer-II /IV Corrosion-Introduction, types Mechanism of dry and wet corrosion “Corrosion is defined as the gradual destruction of metals or alloys by the chemical or electrochemical reaction with its environment.” Causes of corrosion occurs Most of the metals (except noble metals) naturally exis ...
Chapter 2: Mass Relations in Formulas, Chemical Reactions, and
... isotopes of the element carbon present in a typical sample on earth. Note: Atomic masses are also called atomic weights. ...
... isotopes of the element carbon present in a typical sample on earth. Note: Atomic masses are also called atomic weights. ...
4) What is the term for the procedure of collecting data and recording
... percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% manganese, what is the mass of manganese in the sample? A) 0.0138 g B) 0.0550 g C) 0.182 g D) 0.727 g E ...
... percent of copper in the sample? A) 12% B) 29% C) 50% D) 58% E) 75% Stainless steel is composed of iron, manganese, chromium, and nickel. If a 2.00 g sample was analyzed and found to contain 2.75% manganese, what is the mass of manganese in the sample? A) 0.0138 g B) 0.0550 g C) 0.182 g D) 0.727 g E ...
cont. - Appoquinimink High School
... • While the element iron is defined as being made up of neutral atoms with 26 protons and 26 electrons, not every iron atom has the same number of neutrons. • Atoms that have the same number of protons but different numbers of neutrons are called isotopes. (cont.) © 2004 Key Curriculum Press. ...
... • While the element iron is defined as being made up of neutral atoms with 26 protons and 26 electrons, not every iron atom has the same number of neutrons. • Atoms that have the same number of protons but different numbers of neutrons are called isotopes. (cont.) © 2004 Key Curriculum Press. ...
Hein and Arena - faculty at Chemeketa
... The concentrations of A, B, C, and D represent the equilibrium concentrations. The brackets around [A], [B], [C], and [D] represent concentrations in Molarity. The products are written on the top of the fraction & the reactants on the bottom. The coefficients to balance the equation a, b, c, and d a ...
... The concentrations of A, B, C, and D represent the equilibrium concentrations. The brackets around [A], [B], [C], and [D] represent concentrations in Molarity. The products are written on the top of the fraction & the reactants on the bottom. The coefficients to balance the equation a, b, c, and d a ...
Factors that affect the rate of reactions
... not used up in the reaction. It does not change the products, it only helps make the products faster. In your body, or in biology, a catalyst is called an____________. There are 1000’s of enzymes that control everything in your body. Enzymes are large organic molecules. Ex, Saliva, contains the enzy ...
... not used up in the reaction. It does not change the products, it only helps make the products faster. In your body, or in biology, a catalyst is called an____________. There are 1000’s of enzymes that control everything in your body. Enzymes are large organic molecules. Ex, Saliva, contains the enzy ...
Supplemental Informaton
... Noble Gas Notation for Electron Configuration. “Shorthand Notation” (ne-Va-s-p) The noble gas notation for electron configuration is a way of writing the electron configuration for an element using the symbol of the noble gas to represent the “core” inner electrons. Consider the example below for s ...
... Noble Gas Notation for Electron Configuration. “Shorthand Notation” (ne-Va-s-p) The noble gas notation for electron configuration is a way of writing the electron configuration for an element using the symbol of the noble gas to represent the “core” inner electrons. Consider the example below for s ...
1 Course Code– CH1141 Semester – I Credit
... 10. Name a carrier gas used in gas chromatography. 10x1 = 10 marks ...
... 10. Name a carrier gas used in gas chromatography. 10x1 = 10 marks ...
BC Science 10 Workbook Answers
... include introduction of chemicals, toxins, wastes, or micro-organisms into the environment. 6. Overexploitation can result in extinction of a species and a loss of genetic diversity. In turn, the populations are less resistant to disease and less able to adapt to changes in their environment. 7. Tra ...
... include introduction of chemicals, toxins, wastes, or micro-organisms into the environment. 6. Overexploitation can result in extinction of a species and a loss of genetic diversity. In turn, the populations are less resistant to disease and less able to adapt to changes in their environment. 7. Tra ...
Acids - Beck-Shop
... will learn about the atomic masses that you see on your periodic table. You will also learn about the special code of chemistry: the formulae, and equations that allow chemists to communicate. Amount of substance and its unit the mole, provides chemists with an ability to convert between mass, conce ...
... will learn about the atomic masses that you see on your periodic table. You will also learn about the special code of chemistry: the formulae, and equations that allow chemists to communicate. Amount of substance and its unit the mole, provides chemists with an ability to convert between mass, conce ...
Subject Materials for Chemistry
... oxides some of these oxides escape into atmosphere and rest forms slag. This slag is skimmed off. When all the impurities are removed, required amount of carbon is added to molten iron to form steel of our choice. The following reactions take place in Bessemer converter. 2Mn + O2 ...
... oxides some of these oxides escape into atmosphere and rest forms slag. This slag is skimmed off. When all the impurities are removed, required amount of carbon is added to molten iron to form steel of our choice. The following reactions take place in Bessemer converter. 2Mn + O2 ...