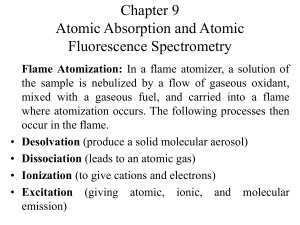
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... Fig. 9-10a shows the emission spectrum of a typical atomic lamp source. With a suitable filter or monochromator, all but one of these lines are removed. Fig. 9-10b shows the absorption spectrum for the analyte between wavelengths 1 and 2. Passage of the line from the source through the flame redu ...
... Fig. 9-10a shows the emission spectrum of a typical atomic lamp source. With a suitable filter or monochromator, all but one of these lines are removed. Fig. 9-10b shows the absorption spectrum for the analyte between wavelengths 1 and 2. Passage of the line from the source through the flame redu ...
Quarter 1
... 1. The average atomic mass of Chlorine is 35.453 amu. The isotopes of Chlorine are Chlorine35 and Chlorine-37. Determine which isotope will be found in greatest abundance given the atomic mass. Answer: Chlorine exists as two common isotopes. Chlorine-35 has an atomic mass of about 35 amu, Chlorine-3 ...
... 1. The average atomic mass of Chlorine is 35.453 amu. The isotopes of Chlorine are Chlorine35 and Chlorine-37. Determine which isotope will be found in greatest abundance given the atomic mass. Answer: Chlorine exists as two common isotopes. Chlorine-35 has an atomic mass of about 35 amu, Chlorine-3 ...
Chemical and physical changes
... A. ……………. changes are those in which the substances continue ............... the same ones. B. Chemical ……………….. are those in which the ……………….. that there are at the beginning ……………….. and in their place new ones appear. C. The ……………….. changes are called chemical ……………….. . D. Pure substances can ...
... A. ……………. changes are those in which the substances continue ............... the same ones. B. Chemical ……………….. are those in which the ……………….. that there are at the beginning ……………….. and in their place new ones appear. C. The ……………….. changes are called chemical ……………….. . D. Pure substances can ...
Chemical Reactivity as Described by Quantum Chemical Methods
... aspects can be included when further linking reactivity to the property vertex. In the field of organic chemistry, the ab initio calculation of functional group properties and their use in studies on acidity and basicity is discussed together with the use of DFT descriptors to study the kinetics of ...
... aspects can be included when further linking reactivity to the property vertex. In the field of organic chemistry, the ab initio calculation of functional group properties and their use in studies on acidity and basicity is discussed together with the use of DFT descriptors to study the kinetics of ...
06 Electronic Structure
... • As the number of electrons increases, so does the repulsion between them. • Therefore, in atoms with more than one electron, not all orbitals on the same energy level are degenerate. • Orbital sets in the same sublevel are still degenerate. • Energy levels start to overlap in energy (e.g., 4s is l ...
... • As the number of electrons increases, so does the repulsion between them. • Therefore, in atoms with more than one electron, not all orbitals on the same energy level are degenerate. • Orbital sets in the same sublevel are still degenerate. • Energy levels start to overlap in energy (e.g., 4s is l ...
Development of Novel Catalytic Asymmetric Reactions using
... of late transition metal enolates, especially palladium. The electronegativity of late transition metals being significantly greater than that of the alkali metals in conventional enolates; the extent of enolate polarization in the former case is expected to be smaller. Therefore, late transition me ...
... of late transition metal enolates, especially palladium. The electronegativity of late transition metals being significantly greater than that of the alkali metals in conventional enolates; the extent of enolate polarization in the former case is expected to be smaller. Therefore, late transition me ...
Example 1-2
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
- Pittsfield High School
... Three rules—the aufbau principle, the Pauli exclusion principle, and Hund’s rule—tell you how to find the electron configurations of atoms. ...
... Three rules—the aufbau principle, the Pauli exclusion principle, and Hund’s rule—tell you how to find the electron configurations of atoms. ...
Basics of Chemistry
... *1 amu = 1.6605 × 10 g (We will discuss “amu” as we proceed further in this section) The atomic number (symbol is Z) of an element is defined as the number of protons in the nucleus. In the periodic table, elements are arranged in order of increasing atomic numbers. A molecule is the smallest partic ...
... *1 amu = 1.6605 × 10 g (We will discuss “amu” as we proceed further in this section) The atomic number (symbol is Z) of an element is defined as the number of protons in the nucleus. In the periodic table, elements are arranged in order of increasing atomic numbers. A molecule is the smallest partic ...
Molar mass
... Scientists use the mole as a unit of measure • AMU is not practical to use • Can’t measure individual atoms very easily • Measuring in grams is desirable • Ratio of atoms is transferred between amu and grams ...
... Scientists use the mole as a unit of measure • AMU is not practical to use • Can’t measure individual atoms very easily • Measuring in grams is desirable • Ratio of atoms is transferred between amu and grams ...
Welcome to AP Chemistry
... always low. For example: A balance could have a defect that caused it to read 1.000 g too high every time. A thermometer could have a defect that cased it to read 1.00 degrees C too low every time. 1. Would using the same balance every time in an experiment be important? Why or why not? 2. In an exp ...
... always low. For example: A balance could have a defect that caused it to read 1.000 g too high every time. A thermometer could have a defect that cased it to read 1.00 degrees C too low every time. 1. Would using the same balance every time in an experiment be important? Why or why not? 2. In an exp ...
Chapter 2
... All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department, Harcourt, Inc. 6277 Sea Harbor Drive, Orlando, Florida 32887-6777 Copyright © 2001 by Harcourt, Inc. All rights reserved. ...
... All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department, Harcourt, Inc. 6277 Sea Harbor Drive, Orlando, Florida 32887-6777 Copyright © 2001 by Harcourt, Inc. All rights reserved. ...
Welcome to AP Chemistry
... always low. For example: A balance could have a defect that caused it to read 1.000 g too high every time. A thermometer could have a defect that cased it to read 1.00 degrees C too low every time. 1. Would using the same balance every time in an experiment be important? Why or why not? 2. In an exp ...
... always low. For example: A balance could have a defect that caused it to read 1.000 g too high every time. A thermometer could have a defect that cased it to read 1.00 degrees C too low every time. 1. Would using the same balance every time in an experiment be important? Why or why not? 2. In an exp ...
Chapter 3 Stoichiometry
... Stoichiometry: The study of quantities of materials consumed and produced in chemical reactions Atomic Masses: Are determined by comparing with 12C (carbon-12 scale). By definition, carbon-12 is assigned a mass of exactly 12 atomic mass units (amu) and the masses of all other atoms are given relativ ...
... Stoichiometry: The study of quantities of materials consumed and produced in chemical reactions Atomic Masses: Are determined by comparing with 12C (carbon-12 scale). By definition, carbon-12 is assigned a mass of exactly 12 atomic mass units (amu) and the masses of all other atoms are given relativ ...
AP Chemistry
... The mass of an aqueous solution of H2O2 is 6.951 g. The H2O2 in the solution decomposes completely according to the reaction represented below. The O2(g) produced is collected in an inverted graduated tube over water at 23.4oC and has a volume of 182.4 mL when the water levels inside and outside of ...
... The mass of an aqueous solution of H2O2 is 6.951 g. The H2O2 in the solution decomposes completely according to the reaction represented below. The O2(g) produced is collected in an inverted graduated tube over water at 23.4oC and has a volume of 182.4 mL when the water levels inside and outside of ...
Atoms and Elements
... In 1808, John Dalton explained the laws just discussed with his atomic theory, which included the following concepts: 1. Each element is composed of tiny, indestructible particles called atoms. 2. All atoms of a given element have the same mass and other properties that distinguish them from the ato ...
... In 1808, John Dalton explained the laws just discussed with his atomic theory, which included the following concepts: 1. Each element is composed of tiny, indestructible particles called atoms. 2. All atoms of a given element have the same mass and other properties that distinguish them from the ato ...
ism ismismismismismrapidrevisionquestionsismismismismismism
... Osmosis is defined as the process by which solvent particles move from a solution of lower concentration to a solution of higher concentration through a semi- permeable membrane. Osmotic pressure is defined as the excess pressure that must be applied to the concentrated solution side to prevent the ...
... Osmosis is defined as the process by which solvent particles move from a solution of lower concentration to a solution of higher concentration through a semi- permeable membrane. Osmotic pressure is defined as the excess pressure that must be applied to the concentrated solution side to prevent the ...
Physical Science e
... Each book in the Power Practice™ series contains over 100 ready-to-use activity pages to provide students with skill practice. The fun activities can be used to supplement and enhance what you are teaching in your classroom. Give an activity page to students as independent class work, or send the pa ...
... Each book in the Power Practice™ series contains over 100 ready-to-use activity pages to provide students with skill practice. The fun activities can be used to supplement and enhance what you are teaching in your classroom. Give an activity page to students as independent class work, or send the pa ...
Document
... Thermochemical Equations with Energy Terms You are already familiar, from your grade 11 Chemistry course, with the first way to describe the enthalpy change in a chemical reaction: include it as a term in a thermochemical equation. If a reaction is endothermic, it requires a certain quantity of ener ...
... Thermochemical Equations with Energy Terms You are already familiar, from your grade 11 Chemistry course, with the first way to describe the enthalpy change in a chemical reaction: include it as a term in a thermochemical equation. If a reaction is endothermic, it requires a certain quantity of ener ...
Chapter 1 Introduction
... compared, it will usually be found that the sizes of the characteristic plane ―faces‖ are not in the same proportion the ―habit‖ varies from crystal to crystal‖. On the other hand, the interfacial angles are always the same for crystals of a given material. ...
... compared, it will usually be found that the sizes of the characteristic plane ―faces‖ are not in the same proportion the ―habit‖ varies from crystal to crystal‖. On the other hand, the interfacial angles are always the same for crystals of a given material. ...
BASIC CHEMICAL CONCEPTS
... to this question, but by the colour criterion for differentiating substances,10 the second answer is surely correct (i.e. mercury vapour is a different substance from liquid mercury).11 The above discussion relates to coloured substances. It can be extended to colourless ones by analogy.12 If a colo ...
... to this question, but by the colour criterion for differentiating substances,10 the second answer is surely correct (i.e. mercury vapour is a different substance from liquid mercury).11 The above discussion relates to coloured substances. It can be extended to colourless ones by analogy.12 If a colo ...
chapter_4_notes
... Was a young Danish physicist and a student of Rutherford’s when he proposed that the electron is only found in specific circular paths, or orbits, around the nucleus. This model of the atom is similar to the solar system because the electrons orbit the nucleus like the planets orbit the sun. Each el ...
... Was a young Danish physicist and a student of Rutherford’s when he proposed that the electron is only found in specific circular paths, or orbits, around the nucleus. This model of the atom is similar to the solar system because the electrons orbit the nucleus like the planets orbit the sun. Each el ...
Notes mole molar mass ions compounds
... chemical reaction to form magnesium oxide is: 2Mg(s) + O2(g) → 2MgO(s) Some approaches to answer this question will be developed in future weeks. Common approaches to solve this question involve the Law of Conservation of Mass (not enough data given above), the Law of Definite Proportions, and stoic ...
... chemical reaction to form magnesium oxide is: 2Mg(s) + O2(g) → 2MgO(s) Some approaches to answer this question will be developed in future weeks. Common approaches to solve this question involve the Law of Conservation of Mass (not enough data given above), the Law of Definite Proportions, and stoic ...























