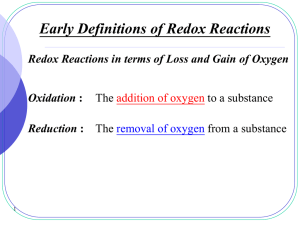
Redox reactions - SALEM-Immanuel Lutheran College
... bond (including ionic bond) between two unlike atoms is presumed to belong to the more electronegative atom. ...
... bond (including ionic bond) between two unlike atoms is presumed to belong to the more electronegative atom. ...
Covalent Bonding and Molecular Structure
... Interactive Figure 8.1.1 shows a demonstration of Coulomb’s law using a gold leaf electroscope in which a narrow metal plate and a thin sheet of gold are connected to a conducting rod. The plastic pen held above the electroscope carries a static charge, which is transferred to the metals via inducta ...
... Interactive Figure 8.1.1 shows a demonstration of Coulomb’s law using a gold leaf electroscope in which a narrow metal plate and a thin sheet of gold are connected to a conducting rod. The plastic pen held above the electroscope carries a static charge, which is transferred to the metals via inducta ...
Table of Contents - slccscience`s Home Page
... and its compounds. Organic chemistry is the study of carbon and its compounds. Since there are 117 known elements, it often seems odd that an entire branch of chemistry is devoted to a single element and its compounds while the other 116 elements and their compounds are all lumped together in a sepa ...
... and its compounds. Organic chemistry is the study of carbon and its compounds. Since there are 117 known elements, it often seems odd that an entire branch of chemistry is devoted to a single element and its compounds while the other 116 elements and their compounds are all lumped together in a sepa ...
Exercise II
... Finally, the diagram gives information about the rate of a reaction. The higher the energy of the transition state (corresponding to an increase in activation energy Ea ) the slower the reaction is likely to proceed. ...
... Finally, the diagram gives information about the rate of a reaction. The higher the energy of the transition state (corresponding to an increase in activation energy Ea ) the slower the reaction is likely to proceed. ...
Unit 4, Lesson #3 - Patterson Science
... temperatures, until the system no longer changes. At this point, they measure the amounts of both the reactants and products. Just as chemists monitor changes in pH, colour, gas pressure or conductivity of solutions to calculate the rate of a reaction, measuring these values can also be used to calc ...
... temperatures, until the system no longer changes. At this point, they measure the amounts of both the reactants and products. Just as chemists monitor changes in pH, colour, gas pressure or conductivity of solutions to calculate the rate of a reaction, measuring these values can also be used to calc ...
- Vijay Education Academy
... 36. A compound consisting of the monovalent ions A , B crystallizes in the body centred cubic lattice. (i) What is the formula of the compound? (ii) If one of A+ ions from the corner is replaced by a monovalent ion C +, what would be the simplest formula of the resulting compound? 37. Maneesh, a stu ...
... 36. A compound consisting of the monovalent ions A , B crystallizes in the body centred cubic lattice. (i) What is the formula of the compound? (ii) If one of A+ ions from the corner is replaced by a monovalent ion C +, what would be the simplest formula of the resulting compound? 37. Maneesh, a stu ...
The science of chemistry is concerned with the composition
... the term submicroscopic really ought to be used, because most atoms and molecules are much too small to be seen even under a microscope.) In any event, chemists continually try to explain the macroscopic world in microscopic terms. ...
... the term submicroscopic really ought to be used, because most atoms and molecules are much too small to be seen even under a microscope.) In any event, chemists continually try to explain the macroscopic world in microscopic terms. ...
H - sintak
... Since both graphite and oxygen are stable allotrophic forms, ∆H°ƒ (C, graphite) and ∆H°ƒ (O2, g) are zero. ∆H°rxn = (1mol) ∆H°ƒ (CO2, g) = -393.5 kJ ∆H°ƒ (CO2, g) = -393.5 kJ/mol ...
... Since both graphite and oxygen are stable allotrophic forms, ∆H°ƒ (C, graphite) and ∆H°ƒ (O2, g) are zero. ∆H°rxn = (1mol) ∆H°ƒ (CO2, g) = -393.5 kJ ∆H°ƒ (CO2, g) = -393.5 kJ/mol ...
The science of chemistry is concerned with the
... the term submicroscopic really ought to be used, because most atoms and molecules are much too small to be seen even under a microscope.) In any event, chemists continually try to explain the macroscopic world in microscopic terms. ...
... the term submicroscopic really ought to be used, because most atoms and molecules are much too small to be seen even under a microscope.) In any event, chemists continually try to explain the macroscopic world in microscopic terms. ...
Reaction of niobium with water
... panchromium ("something which can take or have any colour"). He later renamed the element erythronium ("red") after noting that most of these salts turned red upon heating. It seems he withdrew his claim after a Frenchman, CollettDesotils, disputed. It was only 30 years later that it was shown that ...
... panchromium ("something which can take or have any colour"). He later renamed the element erythronium ("red") after noting that most of these salts turned red upon heating. It seems he withdrew his claim after a Frenchman, CollettDesotils, disputed. It was only 30 years later that it was shown that ...
l-Changing Collisions in Cold Rydberg Gases
... dependence of this long-lasting electron signal is shown in Fig. 1. There, the dye laser was operated at a low pulse energy (< 1 mJ) and at fixed wavelengths. The similarity of the two depicted results is coincidental. The absolute strength of the signal has been found to depend on the quantum numbe ...
... dependence of this long-lasting electron signal is shown in Fig. 1. There, the dye laser was operated at a low pulse energy (< 1 mJ) and at fixed wavelengths. The similarity of the two depicted results is coincidental. The absolute strength of the signal has been found to depend on the quantum numbe ...
Honors Chemistry Unit 02
... quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms change the way they are bound together with other atoms to fo ...
... quite), but atoms of different elements have different masses. – Atoms combine in simple, whole-number ratios to form compounds. – Atoms of one element cannot change into atoms of another element (not quite). In a chemical reaction, atoms change the way they are bound together with other atoms to fo ...
L-11 Chemical thermodynamics
... Change in state of a system is defined by giving the initial and the final state of the system. We can understand it by considering another example. We travel from one point to another. The distance travelled depends on the path or the route we take. But the separation between these two points on th ...
... Change in state of a system is defined by giving the initial and the final state of the system. We can understand it by considering another example. We travel from one point to another. The distance travelled depends on the path or the route we take. But the separation between these two points on th ...
231. - Department of Chemistry
... In this study we report the completion of measurements of the kinetics and energetics of the ligation of (c-C5H5)Fe⫹ in He bath gas at 0.35 Torr with a variety of inorganic ligands containing hydrogen, carbon, nitrogen, and oxygen. Previous measurements in our laboratory have shown that the presence ...
... In this study we report the completion of measurements of the kinetics and energetics of the ligation of (c-C5H5)Fe⫹ in He bath gas at 0.35 Torr with a variety of inorganic ligands containing hydrogen, carbon, nitrogen, and oxygen. Previous measurements in our laboratory have shown that the presence ...
semester i - Pt. Ravishankar Shukla University
... Determination of the rate constant for the decomposition of hydrogen peroxide by Fe and Cu ions. Determination of the primary salt effect on the kinetics of ionic reactions and testing of the Bronsted relationship (iodide ion is oxidized by persulphate ion). ...
... Determination of the rate constant for the decomposition of hydrogen peroxide by Fe and Cu ions. Determination of the primary salt effect on the kinetics of ionic reactions and testing of the Bronsted relationship (iodide ion is oxidized by persulphate ion). ...
Chemistry Skills Practice Assignments
... a) How many protons does this atom have? b) What is the identity of this atom? c) How many neutrons does this atom have? 2. What is an isotope? Give an example. 3. A certain ion has an atomic number of 16, a mass number of 33, and 18 electrons. a) What is the charge on the ion? b) What is the identi ...
... a) How many protons does this atom have? b) What is the identity of this atom? c) How many neutrons does this atom have? 2. What is an isotope? Give an example. 3. A certain ion has an atomic number of 16, a mass number of 33, and 18 electrons. a) What is the charge on the ion? b) What is the identi ...
The Mole: A Measurement of Matter
... You live in a quantitative world. The grade you got on your last exam, the number of times you heard your favorite song on the radio yesterday, and the cost of a bicycle you would like to own are all important quantities to you. These are quantities that answer questions such as "How much?" or "How ...
... You live in a quantitative world. The grade you got on your last exam, the number of times you heard your favorite song on the radio yesterday, and the cost of a bicycle you would like to own are all important quantities to you. These are quantities that answer questions such as "How much?" or "How ...
D--All Websites-eChemistryHelp-.mdi
... 1. The definition : Oxidation number of an element in a particular compound represents the number of electrons lost or gained by an element during its change from free state into that compound or Oxidation number of an element in a particular compound represents the extent of oxidation or reduction ...
... 1. The definition : Oxidation number of an element in a particular compound represents the number of electrons lost or gained by an element during its change from free state into that compound or Oxidation number of an element in a particular compound represents the extent of oxidation or reduction ...
Holt Modern Chemistry Workbook
... The Octet Rule As the diagram at the right shows, the overlapping orbital in a hydrogen molecule contains two electrons. This is the same electron configuration as in a helium atom, which is a stable noble gas. Each hydrogen atom in the molecule is said to have a noble-gas configuration, since its o ...
... The Octet Rule As the diagram at the right shows, the overlapping orbital in a hydrogen molecule contains two electrons. This is the same electron configuration as in a helium atom, which is a stable noble gas. Each hydrogen atom in the molecule is said to have a noble-gas configuration, since its o ...
Chap 05A-Chemical Bonds.pptx
... q Most elements, except noble gases, combine to form compounds. Compounds are the result of the formation of chemical bonds between two or more different elements. q In the formation of a chemical bond, atoms lose, gain or share valence electrons to complete their outer shell and attain a noble ...
... q Most elements, except noble gases, combine to form compounds. Compounds are the result of the formation of chemical bonds between two or more different elements. q In the formation of a chemical bond, atoms lose, gain or share valence electrons to complete their outer shell and attain a noble ...