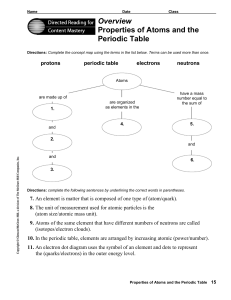
Overview Properties of Atoms and the Periodic Table
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called ...
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called ...
Chapter 5: Atomic Structure
... • Democritus (460-400B.C.) first suggested the existence of these particles, which he called “atoms” for the Greek word for “uncuttable”. They lacked experimental support due to the lack of scientific testing at the time. • John Dalton (1766-1844) performed experiments to study the ratios in which e ...
... • Democritus (460-400B.C.) first suggested the existence of these particles, which he called “atoms” for the Greek word for “uncuttable”. They lacked experimental support due to the lack of scientific testing at the time. • John Dalton (1766-1844) performed experiments to study the ratios in which e ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
... same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
... same family, they share similar characteristics. Each element family has a unique name as well! Let’s look at them now… ...
Atomic Structure of hydrogen
... Cancer treatment – A weak beam of radiation will kill cancer cells more readily than healthy cells. Carbon dating – All living things contain a known proportion of radioactive carbon-14 atoms. When an organism dies it stops taking in new carbon atoms so the proportion of carbon-14 atoms slowly drops ...
... Cancer treatment – A weak beam of radiation will kill cancer cells more readily than healthy cells. Carbon dating – All living things contain a known proportion of radioactive carbon-14 atoms. When an organism dies it stops taking in new carbon atoms so the proportion of carbon-14 atoms slowly drops ...
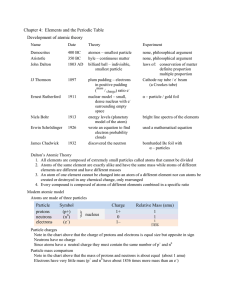
Chapter 4: Elements and the Periodic Table Development of atomic
... Actinides – the bottom row of which only Ac, Th, Pa, and U occur naturally on earth Most of the actinides are synthetic elements formed in particle accelerators Mixed group metals – metals found in the bottom left corner of the p block The most familiar of these metals are Al, Sn, and Pb Pb was used ...
... Actinides – the bottom row of which only Ac, Th, Pa, and U occur naturally on earth Most of the actinides are synthetic elements formed in particle accelerators Mixed group metals – metals found in the bottom left corner of the p block The most familiar of these metals are Al, Sn, and Pb Pb was used ...
Honors Unit 5 Practice Test
... a. sodium has four or five electrons. b. the atomic radius has increased. c. a d electron has been removed. d. the noble gas configuration has been reached. Which is the best reason that the atomic radius generally increases with atomic number in each group of elements? a. The nuclear charge increas ...
... a. sodium has four or five electrons. b. the atomic radius has increased. c. a d electron has been removed. d. the noble gas configuration has been reached. Which is the best reason that the atomic radius generally increases with atomic number in each group of elements? a. The nuclear charge increas ...
Nature of Matter
... • Proton: +, nucleus • Neutrons: neutral, nucleus • Electrons: negative, cloud around nucleus; organization inside cloud – into shells ...
... • Proton: +, nucleus • Neutrons: neutral, nucleus • Electrons: negative, cloud around nucleus; organization inside cloud – into shells ...
Matt Knorr 2/3/2014 Summary: This lesson will explore the smallest
... 5-10 minutes pass out handout on basic meanings and derivations of the vocabulary words. Test students’ intuitions on guessing definitions of other words in physics and chemistry, namely “neutrino”, meaning small neutral particle, “nucleon”, meaning particles in the nucleus, or “prototype”, meaning ...
... 5-10 minutes pass out handout on basic meanings and derivations of the vocabulary words. Test students’ intuitions on guessing definitions of other words in physics and chemistry, namely “neutrino”, meaning small neutral particle, “nucleon”, meaning particles in the nucleus, or “prototype”, meaning ...
8th-interlude-for-atoms - Epiphany Catholic School
... Compounds Formed when atoms of more than one element combined. Properties of a compound are very different from properties of the elements that make it up. Ex. NaCl = salt ...
... Compounds Formed when atoms of more than one element combined. Properties of a compound are very different from properties of the elements that make it up. Ex. NaCl = salt ...
Covalent Bonds
... indicated by the number of protons added to the number of neutrons • The atomic mass indicates approximately how much matter it contains as compared with another atom • Atomic mass number is denoted by a superscript to the left of the chemical symbol atomic mass ...
... indicated by the number of protons added to the number of neutrons • The atomic mass indicates approximately how much matter it contains as compared with another atom • Atomic mass number is denoted by a superscript to the left of the chemical symbol atomic mass ...
Atomic Theories and Scientists Notes
... Does Not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. Different elements have different atoms YES! 4. Atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merel ...
... Does Not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. Different elements have different atoms YES! 4. Atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merel ...
creator of the atomic theory
... moved in paths at certain distances around the nucleus, called orbitals. Higher orbitals have more energy. When electrons move down orbitals, light (photons) is emitted. ...
... moved in paths at certain distances around the nucleus, called orbitals. Higher orbitals have more energy. When electrons move down orbitals, light (photons) is emitted. ...
3rd Quarter Test
... a) forward reaction stops b) reverse reaction stops c) concentration of the reactants and the products becomes equal d) rates of the opposing reaction becomes equal 20) For a chemical system at equilibrium, a rise in temperature will a) favor the endothermic reaction b) favor the exothermic reaction ...
... a) forward reaction stops b) reverse reaction stops c) concentration of the reactants and the products becomes equal d) rates of the opposing reaction becomes equal 20) For a chemical system at equilibrium, a rise in temperature will a) favor the endothermic reaction b) favor the exothermic reaction ...
Mrs. Jiménez’s Abbreviated Version of Atomic History for Study Purposes
... Discovered the neutrons, which exist so that positively charged protons will not repel each other so as to explode out of the nucleus. Proposed that electrons travel at fixed distances from the nucleus in an atom such that they orbit the nucleus much like planets orbit the sun. He also proposed idea ...
... Discovered the neutrons, which exist so that positively charged protons will not repel each other so as to explode out of the nucleus. Proposed that electrons travel at fixed distances from the nucleus in an atom such that they orbit the nucleus much like planets orbit the sun. He also proposed idea ...
CH 2 Linear
... Democritus (460 – 370 B.C.) proposed that the world is made up of empty space and tiny, invisible particles called atoms. This introduced the atomic theory of matter. Development of Modern Atomic Theory Law of Conservation of Matter (Mass): Lavoisier (1782) observed that in a sealed container, the ...
... Democritus (460 – 370 B.C.) proposed that the world is made up of empty space and tiny, invisible particles called atoms. This introduced the atomic theory of matter. Development of Modern Atomic Theory Law of Conservation of Matter (Mass): Lavoisier (1782) observed that in a sealed container, the ...
1 - shawnschmitt
... f. Hypothesis- a possible explaination for observations, a testable idea g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. metalloid- thos ...
... f. Hypothesis- a possible explaination for observations, a testable idea g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. metalloid- thos ...
Atoms
... Ernest Rutherford – 1911 Gold Foil Experiment ‘As if you had fired a 15 – inch shell at a piece of tissue paper And it came back to hit you.’ ...
... Ernest Rutherford – 1911 Gold Foil Experiment ‘As if you had fired a 15 – inch shell at a piece of tissue paper And it came back to hit you.’ ...
chapter 4 notes
... ___________________________. (identified by the element name followed by the mass # ) ex. C12, C-_____, B-10, B-_____) 22. ________________ _____________ mass is the weighted - average mass of the mixture of all an atoms isotopes. The average atomic mass is close to the mass of its most abundant iso ...
... ___________________________. (identified by the element name followed by the mass # ) ex. C12, C-_____, B-10, B-_____) 22. ________________ _____________ mass is the weighted - average mass of the mixture of all an atoms isotopes. The average atomic mass is close to the mass of its most abundant iso ...
14.2 Notes on Electrons Atoms interact with each other through their
... Atoms interact with each other through their electrons Almost all properties of elements are due to electrons Almost all light you see comes from atoms Each different element has its own characteristic pattern of colors called a spectrum The colors of everything around you come from this property of ...
... Atoms interact with each other through their electrons Almost all properties of elements are due to electrons Almost all light you see comes from atoms Each different element has its own characteristic pattern of colors called a spectrum The colors of everything around you come from this property of ...
4. - period2chem
... Dalton’s billiard ball model-sphere of uniform density. Thomson’s plum pudding model-negative electrons dispersed in positive atom. Rutherford’s nuclear model-dense, positive nucleus surrounded by negative electrons. Bohr’s planetary model-electrons move in circular orbits in specific energy levels. ...
... Dalton’s billiard ball model-sphere of uniform density. Thomson’s plum pudding model-negative electrons dispersed in positive atom. Rutherford’s nuclear model-dense, positive nucleus surrounded by negative electrons. Bohr’s planetary model-electrons move in circular orbits in specific energy levels. ...
SUMMER WORK AP Chemistry
... experiment requires 15.0 g of cyclohexane, whose density at 25 °C is 0.7781 g/mL. What volume of cyclohexane should be used? (c) A spherical ball of lead has a diameter of 5.0 cm. What is the mass of the sphere if lead has a density of 11.34 g.cm3? (The volume of a sphere is (4/3)πr3where r is the r ...
... experiment requires 15.0 g of cyclohexane, whose density at 25 °C is 0.7781 g/mL. What volume of cyclohexane should be used? (c) A spherical ball of lead has a diameter of 5.0 cm. What is the mass of the sphere if lead has a density of 11.34 g.cm3? (The volume of a sphere is (4/3)πr3where r is the r ...