Chapter 2 Law of
... • Nonmetals tend to form anions by gaining electrons. • Meaning, they have more electrons than protons resulting in a negative charge. • Let Let’ss look at the nonmetals in A groups of the periodic table • The negative charge on these nonmetals is usually equal to the number of spaces to the right t ...
... • Nonmetals tend to form anions by gaining electrons. • Meaning, they have more electrons than protons resulting in a negative charge. • Let Let’ss look at the nonmetals in A groups of the periodic table • The negative charge on these nonmetals is usually equal to the number of spaces to the right t ...
CHEM 1411 NAME: PRACTICE EXAM #3 (Chapters 6
... Sodium and potassium have similar chemical and physical properties. This is best explained by the fact that both elements A) are active metals. B) are in Period 1 of the periodic table. C) have the same ground-state valence-electron configuration. D) have low relative atomic masses. E) have relative ...
... Sodium and potassium have similar chemical and physical properties. This is best explained by the fact that both elements A) are active metals. B) are in Period 1 of the periodic table. C) have the same ground-state valence-electron configuration. D) have low relative atomic masses. E) have relative ...
Earth Science - Green Local Schools
... Molecule Atomic mass unit Parts of the atom – nucleus, proton, neutron, electron, Average atomic mass energy levels Metal Valence electron Nonmetal Period Semiconductor Group Alkali metal Ion Alkaline-earth metal Atomic number Transition metal Mass number Noble gases Electron Cloud Model / Bohr Mode ...
... Molecule Atomic mass unit Parts of the atom – nucleus, proton, neutron, electron, Average atomic mass energy levels Metal Valence electron Nonmetal Period Semiconductor Group Alkali metal Ion Alkaline-earth metal Atomic number Transition metal Mass number Noble gases Electron Cloud Model / Bohr Mode ...
Class 9 CBSE Test paper Solved Chapter 3: Structure of...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
Topic 2.1 The Nuclear Atom
... • this is NOT IB material until indicated • it is very interesting from a geeky-science stand point • it will help you understand and appreciate the structure of the atom • you are not responsible for knowing the information from all thescientists ...
... • this is NOT IB material until indicated • it is very interesting from a geeky-science stand point • it will help you understand and appreciate the structure of the atom • you are not responsible for knowing the information from all thescientists ...
1 - Hatboro
... 22. How do you convert from celsius to kelvin? 23. Where on the periodic table are the metals? Metalloids? Nonmetals? Nobel gases? 24. What is Dalton's atomic theory? 25. What is an atomic mass unit? 26. What is the law of Conservation of mass? 27. Describe Rutherford’s experiment and his model of t ...
... 22. How do you convert from celsius to kelvin? 23. Where on the periodic table are the metals? Metalloids? Nonmetals? Nobel gases? 24. What is Dalton's atomic theory? 25. What is an atomic mass unit? 26. What is the law of Conservation of mass? 27. Describe Rutherford’s experiment and his model of t ...
2 Atomic Theory Development of Theory • Historical Atomic Models
... binary ionic compounds the halogens are -1. Otherwise the oxidation number is calculated . example: given, NaClO4, where Na = +1 (Group 1), O = -2, since Na + Cl + 4 O = 0, ...
... binary ionic compounds the halogens are -1. Otherwise the oxidation number is calculated . example: given, NaClO4, where Na = +1 (Group 1), O = -2, since Na + Cl + 4 O = 0, ...
notes: 12 - wvhs.wlwv.k12.or.us
... Rutherford’s “Scattering” Experiment: -positively charged alpha particles (helium nuclei) were shot through a thin gold foil. -most alpha particles passed through the foil, or were deflected through moderate angles. -a few were reflected at extreme angles, or even came shooting right back to the sou ...
... Rutherford’s “Scattering” Experiment: -positively charged alpha particles (helium nuclei) were shot through a thin gold foil. -most alpha particles passed through the foil, or were deflected through moderate angles. -a few were reflected at extreme angles, or even came shooting right back to the sou ...
SCIENCE LONG TEST
... atoms were small, hard particles made of the same material but of different shapes and sizes there were an infinite number of these atoms and they were constantly in motion atoms had the ability to combine with other atoms atoms could no longer be divided into smaller particles The early ideas about ...
... atoms were small, hard particles made of the same material but of different shapes and sizes there were an infinite number of these atoms and they were constantly in motion atoms had the ability to combine with other atoms atoms could no longer be divided into smaller particles The early ideas about ...
How to Assign Oxidation Numbers
... bonded to fluorine (where it may be +1 or +2) and in peroxides where it has an oxidation state of –1 • The sum of the oxidation states of all the atoms in a molecule or ion is equal to the overall charge on the species. ...
... bonded to fluorine (where it may be +1 or +2) and in peroxides where it has an oxidation state of –1 • The sum of the oxidation states of all the atoms in a molecule or ion is equal to the overall charge on the species. ...
The Atomic Nature of Matter
... • Atoms are always moving • It takes 6 years for one of your exhaled breaths to mix evenly with the atmosphere ...
... • Atoms are always moving • It takes 6 years for one of your exhaled breaths to mix evenly with the atmosphere ...
Personal Introduction
... 5.4 Niels Bohr used the quantum hypothesis to explain atomic spectra Max Planck’s quantum hypothesis (量子假设): a beam of light energy is not the continuous stream of energy, but consists of small, discrete packets of energy. Each packet was called a quantum. In 1905, Einstein recognized that these qu ...
... 5.4 Niels Bohr used the quantum hypothesis to explain atomic spectra Max Planck’s quantum hypothesis (量子假设): a beam of light energy is not the continuous stream of energy, but consists of small, discrete packets of energy. Each packet was called a quantum. In 1905, Einstein recognized that these qu ...
Electron Cloud Model-Reading selection
... Electrons are found in clouds that surround the nucleus of an atom. Those clouds are specific distances away from the nucleus and are generally organized into shells. Because electrons move so quickly, it is impossible to see where they are at a specific moment in time. After years of experimentatio ...
... Electrons are found in clouds that surround the nucleus of an atom. Those clouds are specific distances away from the nucleus and are generally organized into shells. Because electrons move so quickly, it is impossible to see where they are at a specific moment in time. After years of experimentatio ...
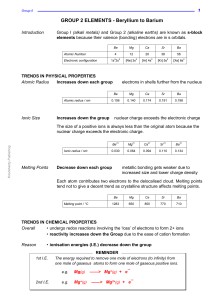
GROUP 2 ELEMENTS - Beryllium to Barium
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
Chapters 19 & 20
... Elements in group 1A through 8A are called representative elements because they display a wide range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative element ...
... Elements in group 1A through 8A are called representative elements because they display a wide range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative element ...
Atomic Structure
... The mass number of this isotope of lithium is 7. Notice that 7 is equal to the total number of protons and neutrons. If you remove the protons (atomic number), the neutrons are left. ...
... The mass number of this isotope of lithium is 7. Notice that 7 is equal to the total number of protons and neutrons. If you remove the protons (atomic number), the neutrons are left. ...
Properties of Atoms and the Periodic Table
... increasing numbers of protons and electrons. One proton and one electron are added to each element as you go across the periodic table from left to right. ...
... increasing numbers of protons and electrons. One proton and one electron are added to each element as you go across the periodic table from left to right. ...
Ch. 3
... Chemical formulas have positive and negative ions when combined have their oxidation numbers equaling zero. ...
... Chemical formulas have positive and negative ions when combined have their oxidation numbers equaling zero. ...
7th Grade
... surroundings. This is an endothermic reaction. The temperature of the solution falls to about 35 F for 10 to 15 minutes. ...
... surroundings. This is an endothermic reaction. The temperature of the solution falls to about 35 F for 10 to 15 minutes. ...