Equation Chapter 1 Section 1 Tips for Studying: Take responsibility
... Release Energy, usually in the form of heat! C(s) + O2(g) ...
... Release Energy, usually in the form of heat! C(s) + O2(g) ...
A = Atomic Number
... *Because the mass number is different for each isotope of hydrogen, the number of neutrons in each atom is different. The number of protons, however, is the same. ...
... *Because the mass number is different for each isotope of hydrogen, the number of neutrons in each atom is different. The number of protons, however, is the same. ...
g - Santa Rosa Junior College
... • The nitrogen cycle involves a direct interaction between land and sea. • Atmospheric N2 must be fixed to enter the land and sea. – Atmospheric fixation occurs when lightning provides the energy for the reaction between N2(g) and O2(g). – Industrial fixation results from the production of NH3, whic ...
... • The nitrogen cycle involves a direct interaction between land and sea. • Atmospheric N2 must be fixed to enter the land and sea. – Atmospheric fixation occurs when lightning provides the energy for the reaction between N2(g) and O2(g). – Industrial fixation results from the production of NH3, whic ...
Understanding the Properties of Elements – Chapter 5
... 2. Name the non-metal ion second. The end of its name will change to “-ide”. 3. If the proportion of one element to the other is not 1:1, a subscript is required to indicate the proportion of the element that is more abundant. e.g. For 1 Magnesium atom, there are 2 chlorine atoms: MgCl2. ...
... 2. Name the non-metal ion second. The end of its name will change to “-ide”. 3. If the proportion of one element to the other is not 1:1, a subscript is required to indicate the proportion of the element that is more abundant. e.g. For 1 Magnesium atom, there are 2 chlorine atoms: MgCl2. ...
For H 2 O
... number minus 8 (beginning with column IVA) 3. if an atom is in an even numbered column, it will have an even number oxidation state; similarly with odd numbered columns 4. maximum change is going to be "8" (magic number) ...
... number minus 8 (beginning with column IVA) 3. if an atom is in an even numbered column, it will have an even number oxidation state; similarly with odd numbered columns 4. maximum change is going to be "8" (magic number) ...
- Los Banos Unified School District
... 1898 Marie Curie Studied uranium and thorium and called their spontaneous decay process "radioactivity". She and her husband Pierre also discovered the radioactive elements polonium and radium. ...
... 1898 Marie Curie Studied uranium and thorium and called their spontaneous decay process "radioactivity". She and her husband Pierre also discovered the radioactive elements polonium and radium. ...
Oct 14th ,2015
... atom in terms of its subatomic particles; isotopes and ions; differentiate between the classification and separation of matter ...
... atom in terms of its subatomic particles; isotopes and ions; differentiate between the classification and separation of matter ...
Chemistry 515 Name: L. S. Curtin Soc. Sec. #: February 8, 1999
... 11) Which of the following statements about Daltons Atomic Theory has been shown to be incorrect? a) b) c) d) e) ...
... 11) Which of the following statements about Daltons Atomic Theory has been shown to be incorrect? a) b) c) d) e) ...
Chapter 6 Electronic Structure of Atoms
... Lothar Meyer independently came to the same conclusion about how elements should be grouped. ...
... Lothar Meyer independently came to the same conclusion about how elements should be grouped. ...
Homework Assignment 1 Key
... Matter is made of tiny indivisible particles called atoms o Atoms are made of smaller particles such as protons, neutrons, and electrons o Atoms can be split (nuclear fission) Atoms of the same element are identical o not true because isotopes were discovered. Isotopes of the same element contain di ...
... Matter is made of tiny indivisible particles called atoms o Atoms are made of smaller particles such as protons, neutrons, and electrons o Atoms can be split (nuclear fission) Atoms of the same element are identical o not true because isotopes were discovered. Isotopes of the same element contain di ...
PHY–309 L. Solutions for homework set # 10. Textbook question Q
... This wikipedia page shows a diagram of a color CRT tube. In any CRT tube — a TV, a monitor, an oscilloscope, or an X-ray tube — a beam of electrons hits the anode (a screen, or just a piece of metal) at high speed. When the atoms in the anode are hit by fast electrons, sometimes the inner electrons ...
... This wikipedia page shows a diagram of a color CRT tube. In any CRT tube — a TV, a monitor, an oscilloscope, or an X-ray tube — a beam of electrons hits the anode (a screen, or just a piece of metal) at high speed. When the atoms in the anode are hit by fast electrons, sometimes the inner electrons ...
Atomic Theory Notes
... Why aren’t electrons accounted for in the calculation of the atomic mass? • Electrons are small! • It takes almost 2,000 electrons to equal the mass of one proton or neutron • Electrons are assumed to have a mass of 0 amu ...
... Why aren’t electrons accounted for in the calculation of the atomic mass? • Electrons are small! • It takes almost 2,000 electrons to equal the mass of one proton or neutron • Electrons are assumed to have a mass of 0 amu ...
Atomic Structure -
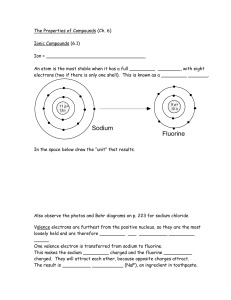
... Because valence electrons are on the last shell, they are the ones that are furthest from the positive field of the protons. Valance electrons are the electrons that determine if an atom can bond with another atom. This means that the valence electrons could be attracted to the nucleus of more posit ...
... Because valence electrons are on the last shell, they are the ones that are furthest from the positive field of the protons. Valance electrons are the electrons that determine if an atom can bond with another atom. This means that the valence electrons could be attracted to the nucleus of more posit ...
Chemistry You Need to Know
... Atoms are mostly empty space Electrons (the smaller particles) were the cause of the small deflections There must be a small area of the atom with most of its mass (the protons) that caused the reflections. He called this small, dense area the nucleus ...
... Atoms are mostly empty space Electrons (the smaller particles) were the cause of the small deflections There must be a small area of the atom with most of its mass (the protons) that caused the reflections. He called this small, dense area the nucleus ...
Nomenclature and chemical reactions PPT
... number minus 8 (beginning with column IVA) 3. if an atom is in an even numbered column, it will have an even number oxidation state; similarly with odd numbered columns 4. maximum change is going to be "8" (magic number) ...
... number minus 8 (beginning with column IVA) 3. if an atom is in an even numbered column, it will have an even number oxidation state; similarly with odd numbered columns 4. maximum change is going to be "8" (magic number) ...
Johnston Middle School Lesson Plan 2015-2016
... Drawing Bohr models and Lewis Structures of the first 18 elements ...
... Drawing Bohr models and Lewis Structures of the first 18 elements ...
Chapter 2.4 Periodic properties of the elements
... can determine it by measuring the distance between the nuclei of bonded, neighbouring atoms. For example, for metals, atomic radius is half the distance between neighbouring nuclei in a crystal of the metal element. For elements that occur as molecules, which is the case for many nonmetals, atomic r ...
... can determine it by measuring the distance between the nuclei of bonded, neighbouring atoms. For example, for metals, atomic radius is half the distance between neighbouring nuclei in a crystal of the metal element. For elements that occur as molecules, which is the case for many nonmetals, atomic r ...
Chapter 4 - Bismuth221
... Third Postulate • Atoms of different elements can physically mix together or can chemically combine in simple whole number ratios to form compounds – Remember when elements mix they do not have to form compounds ...
... Third Postulate • Atoms of different elements can physically mix together or can chemically combine in simple whole number ratios to form compounds – Remember when elements mix they do not have to form compounds ...
atoms = building blocks
... • Matter- the stuff that makes up everything in the universe • Element- A substance that cannot be broken down into other substances by chemical or physical means ...
... • Matter- the stuff that makes up everything in the universe • Element- A substance that cannot be broken down into other substances by chemical or physical means ...