General Concepts
... If you are not given this symbol with the mass number, you can still determine the mass number for the most abundant isotopic form for that atom. For example, if you look on the periodic table for Carbon, you will see a value underneath its symbol - the RELATIVE ATOMIC MASS of 12.011. This mass is ...
... If you are not given this symbol with the mass number, you can still determine the mass number for the most abundant isotopic form for that atom. For example, if you look on the periodic table for Carbon, you will see a value underneath its symbol - the RELATIVE ATOMIC MASS of 12.011. This mass is ...
Notes 2 Balancing
... try different coefficients. Watch for elements whose atoms become unbalanced as a result of your work. • Try the odd-even technique (double the odd number) if you see an even number of a particular atom on one side of an equation and an odd number of that atom on the other side. ...
... try different coefficients. Watch for elements whose atoms become unbalanced as a result of your work. • Try the odd-even technique (double the odd number) if you see an even number of a particular atom on one side of an equation and an odd number of that atom on the other side. ...
- Science
... Models are often used for things that are too small or too large to be observed or that are too difficult to be understood easily ...
... Models are often used for things that are too small or too large to be observed or that are too difficult to be understood easily ...
ppt - Discover Earth Science
... Each electron does not swarm around the nucleus randomly, rather electrons move in definite orbits around the nucleus These orbits are located at specific distances from the nucleus These orbits are called energy ...
... Each electron does not swarm around the nucleus randomly, rather electrons move in definite orbits around the nucleus These orbits are located at specific distances from the nucleus These orbits are called energy ...
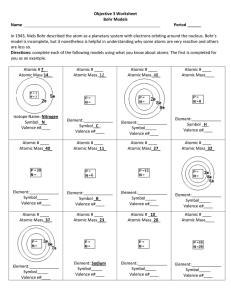
Objective 3 Worksheet Bohr Models Name Period In 1943, Niels
... Objective 3 Worksheet Bohr Models Name ...
... Objective 3 Worksheet Bohr Models Name ...
Test Objectives: Unit 1 – Measurement
... Given the mass of reactants or products be able to determine the unknown mass Be able to define what forms of matter are known as pure substances Be able to distinguish between elements, compounds, & mixtures in terms of their ability to be broken down or separated by physical or chemical means Be a ...
... Given the mass of reactants or products be able to determine the unknown mass Be able to define what forms of matter are known as pure substances Be able to distinguish between elements, compounds, & mixtures in terms of their ability to be broken down or separated by physical or chemical means Be a ...
Review Unit 5
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
Chemistry of Life
... Get into Groups and determine Roles: In each group there are three rolls: player, clue giver, and teleprompter. The clue giver and player sit facing each other, with the teleprompter standing behind the player, displaying the fact cards one at a time to the clue giver. The clue giver reads the facts ...
... Get into Groups and determine Roles: In each group there are three rolls: player, clue giver, and teleprompter. The clue giver and player sit facing each other, with the teleprompter standing behind the player, displaying the fact cards one at a time to the clue giver. The clue giver reads the facts ...
Atomic Theory Overview
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
The Chemistry of Life
... Macromolecules Many of the carbon molecules in living things are so large they are called macromolecules. Macromolecules form by polymerization, in which smaller units called monomers join together to form polymers. Biochemists sort the macromolecules in living things into groups based on their chem ...
... Macromolecules Many of the carbon molecules in living things are so large they are called macromolecules. Macromolecules form by polymerization, in which smaller units called monomers join together to form polymers. Biochemists sort the macromolecules in living things into groups based on their chem ...
Atoms, Elements, and Ions
... Does not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have different atoms YES! 4. atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merel ...
... Does not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have different atoms YES! 4. atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merel ...
The Chemistry of Life
... Macromolecules Many of the carbon molecules in living things are so large they are called macromolecules. Macromolecules form by polymerization, in which smaller units called monomers join together to form polymers. Biochemists sort the macromolecules in living things into groups based on their chem ...
... Macromolecules Many of the carbon molecules in living things are so large they are called macromolecules. Macromolecules form by polymerization, in which smaller units called monomers join together to form polymers. Biochemists sort the macromolecules in living things into groups based on their chem ...
atomic number
... By the late 18th century, scientists finally came to the conclusion that Oxygen was truly an element (can’t be broken down into simpler forms without losing its properties) ...
... By the late 18th century, scientists finally came to the conclusion that Oxygen was truly an element (can’t be broken down into simpler forms without losing its properties) ...
Ch. 3 - My CCSD
... By the late 18th century, scientists finally came to the conclusion that Oxygen was truly an element (can’t be broken down into simpler forms without losing its properties) ...
... By the late 18th century, scientists finally came to the conclusion that Oxygen was truly an element (can’t be broken down into simpler forms without losing its properties) ...
Polar Covalent bonds
... Ionic Compounds •Ionic compounds are composed of both metals and non-metals. The bond that is formed is based on electrostatic forces between negatively(anion) and positively(cation) charged ions. •Ionic bonding occurs by the transfer of one or more electrons from one atom to another ...
... Ionic Compounds •Ionic compounds are composed of both metals and non-metals. The bond that is formed is based on electrostatic forces between negatively(anion) and positively(cation) charged ions. •Ionic bonding occurs by the transfer of one or more electrons from one atom to another ...
Chapter 2/Unit 2: Matter is Made of Atoms
... sheet were mostly open space. Atoms were not a pudding filled with a positively charged material. • Rutherford concluded that an atom had a small, dense, positively charged center that repelled his positively charged “bullets.” • He called the center of the atom the “nucleus” • The nucleus is tiny c ...
... sheet were mostly open space. Atoms were not a pudding filled with a positively charged material. • Rutherford concluded that an atom had a small, dense, positively charged center that repelled his positively charged “bullets.” • He called the center of the atom the “nucleus” • The nucleus is tiny c ...
Chemistry Study Guide: Year 9 Science Page 1 Read your book C3
... but it had problems. For example, he put iron in the same group as oxygen and sulphur, which are two non-metals. As a result, his table was not accepted by other scientists v. In 1869, just five years after John Newlands put forward his law of octaves, a Russian chemist called Dmitri Mendeleev publi ...
... but it had problems. For example, he put iron in the same group as oxygen and sulphur, which are two non-metals. As a result, his table was not accepted by other scientists v. In 1869, just five years after John Newlands put forward his law of octaves, a Russian chemist called Dmitri Mendeleev publi ...
North Haven Public Schools Curriculum
... Atomic and Molecular Structure – The periodic table displays the elements in incerasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. The nucleus of the atom is much smaller than the atom, yet contains most of its mass. ...
... Atomic and Molecular Structure – The periodic table displays the elements in incerasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. The nucleus of the atom is much smaller than the atom, yet contains most of its mass. ...
Atomic Structure
... accounting for more than 99% of the mass of the atom The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
... accounting for more than 99% of the mass of the atom The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
AtomicTimeline
... http://atomictimeline.net/index.php Who stated electron paths cannot be predicted http://wiki.answers.com/Q/Who_stated_electron_paths_cannot_be_predicted ...
... http://atomictimeline.net/index.php Who stated electron paths cannot be predicted http://wiki.answers.com/Q/Who_stated_electron_paths_cannot_be_predicted ...
The nucleus - VCE Chemistry
... some of the anomalies in Mendeleev's table which was based on atomic mass. ...
... some of the anomalies in Mendeleev's table which was based on atomic mass. ...