Chapter 4 Notes
... matter consisted of extremely small particles that could not be divided. • He called these particles atoms from the Greek word atomos, which means “__________” or “________________.” • ___________________ did not think there was a limit to the number of times matter could be divided. • Aristotle tho ...
... matter consisted of extremely small particles that could not be divided. • He called these particles atoms from the Greek word atomos, which means “__________” or “________________.” • ___________________ did not think there was a limit to the number of times matter could be divided. • Aristotle tho ...
Chapter 2 - OrgSites.com
... 14. Describe the relationship between an electron’s potential energy and the distance it is from the nucleus. 15. When an electron absorbs energy, they are called “excited.” When an electron loses energy, it “falls back.” Give an example of each of these processes. ...
... 14. Describe the relationship between an electron’s potential energy and the distance it is from the nucleus. 15. When an electron absorbs energy, they are called “excited.” When an electron loses energy, it “falls back.” Give an example of each of these processes. ...
Isotope Practice Worksheet
... radioactive. Radioactive isotopes emit nuclear radiation in the form of rapidly moving particles or high energy electromagnetic waves. The particles are emitted from the nucleus itself and their removal results in changing the atom from one isotope to another. This change may occur once or emi ...
... radioactive. Radioactive isotopes emit nuclear radiation in the form of rapidly moving particles or high energy electromagnetic waves. The particles are emitted from the nucleus itself and their removal results in changing the atom from one isotope to another. This change may occur once or emi ...
Review for Test
... Write the correct answer on the line. _____ 1. What was concluded about the structure of the atom as a result of the gold-foil experiment? a) A positively charged nucleus is surrounded by positively charged particles b) A positively charged nucleus is surrounded by mostly empty space c) A negatively ...
... Write the correct answer on the line. _____ 1. What was concluded about the structure of the atom as a result of the gold-foil experiment? a) A positively charged nucleus is surrounded by positively charged particles b) A positively charged nucleus is surrounded by mostly empty space c) A negatively ...
name
... NAME _______________________________________ PERIOD _______________ DATE ___________ CHAPTER 5 CHARACTERISTICS OF ELEMENTS Use a periodic table of the elements to help you answer the following questions. 1. a) ...
... NAME _______________________________________ PERIOD _______________ DATE ___________ CHAPTER 5 CHARACTERISTICS OF ELEMENTS Use a periodic table of the elements to help you answer the following questions. 1. a) ...
Noble-ity
... The electrons in the outermost energy shell of an atom are called? Valence electrons You cut a piece of gold in half repeatedly until you end up with the smallest piece that can still be considered gold. What do you have? An ...
... The electrons in the outermost energy shell of an atom are called? Valence electrons You cut a piece of gold in half repeatedly until you end up with the smallest piece that can still be considered gold. What do you have? An ...
Energy and Matter in Chemical Change Science 10
... repetition of characteristics • The success of this model was that it was predictive ...
... repetition of characteristics • The success of this model was that it was predictive ...
I Biology I Lecture Outline Basic Chemistry Life
... attraction between positive and negative ions. 7) Table salJ occurs in soldforln as a 3-dimensionallottice of crystals. 8) When mixed with water, table salt dissolves and the ions separate in the ...
... attraction between positive and negative ions. 7) Table salJ occurs in soldforln as a 3-dimensionallottice of crystals. 8) When mixed with water, table salt dissolves and the ions separate in the ...
Chapter 4 notes - Sussex Regional High School
... Since most of the particles went through, it was mostly empty. Because the pieces turned so much, the positive pieces were heavy. Small volume, big mass, big density. This small dense positive area is the nucleus. There are two regions. The nucleus. With protons and neutrons. Positive charge. Almost ...
... Since most of the particles went through, it was mostly empty. Because the pieces turned so much, the positive pieces were heavy. Small volume, big mass, big density. This small dense positive area is the nucleus. There are two regions. The nucleus. With protons and neutrons. Positive charge. Almost ...
4 Timeline of Structure
... - A calculated negative value only means that light is being emitted during the transition. However, the frequency of that photon of light is still positive. Bohr’s model was important because it introduced the idea of using quantized energy states for electrons in atoms. However, his model is only ...
... - A calculated negative value only means that light is being emitted during the transition. However, the frequency of that photon of light is still positive. Bohr’s model was important because it introduced the idea of using quantized energy states for electrons in atoms. However, his model is only ...
Atomic Structure
... Electrons have a mass of almost zero, which means that the mass of each atom results almost entirely from the number of protons and neutrons in the nucleus. The sum of the protons and neutrons in an atom’s nucleus is the mass number. It is the larger of the two numbers shown in most periodic tables. ...
... Electrons have a mass of almost zero, which means that the mass of each atom results almost entirely from the number of protons and neutrons in the nucleus. The sum of the protons and neutrons in an atom’s nucleus is the mass number. It is the larger of the two numbers shown in most periodic tables. ...
Atomic Theory
... Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Develop lab apparatus / procedures / experimental techniques - alchemists learned how to prepare acids. - developed several alloys - new glassware ...
... Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Develop lab apparatus / procedures / experimental techniques - alchemists learned how to prepare acids. - developed several alloys - new glassware ...
Chemical Reactions and Equations
... or reaction can be confirmed by any or all of the following observations: => change in state => change in colour => change in temperature => evolution of gas. What is a ‘Chemical Equation’? A Chemical Equation represents a chemical reaction. A complete chemical equation represents the reactants, pro ...
... or reaction can be confirmed by any or all of the following observations: => change in state => change in colour => change in temperature => evolution of gas. What is a ‘Chemical Equation’? A Chemical Equation represents a chemical reaction. A complete chemical equation represents the reactants, pro ...
Chemical Reactions and Equations
... or reaction can be confirmed by any or all of the following observations: => change in state => change in colour => change in temperature => evolution of gas. What is a ‘Chemical Equation’? A Chemical Equation represents a chemical reaction. A complete chemical equation represents the reactants, pro ...
... or reaction can be confirmed by any or all of the following observations: => change in state => change in colour => change in temperature => evolution of gas. What is a ‘Chemical Equation’? A Chemical Equation represents a chemical reaction. A complete chemical equation represents the reactants, pro ...
Chapter 8 - Chemical Equations
... Step 1 – Look at the element by itself. Is this element a metal or a nonmetal? Al (aluminum) is a metal because it is located to the left side of the staircase line on the Periodic Table. Step 2 – You will compare the type of element by itself to the similar type of element in the compound. In this ...
... Step 1 – Look at the element by itself. Is this element a metal or a nonmetal? Al (aluminum) is a metal because it is located to the left side of the staircase line on the Periodic Table. Step 2 – You will compare the type of element by itself to the similar type of element in the compound. In this ...
The Building Blocks of Matter
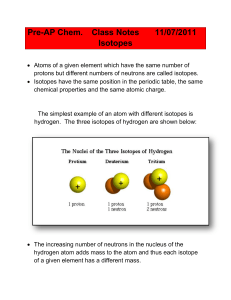
... • Dalton said that every atom of the same element is exactly alike… NOT SO. They do have the same # of protons; however, they do not necessarily have the same # of neutrons! • This means that different atoms of the same element may/will have different masses. • These are: isotopes (atoms having the ...
... • Dalton said that every atom of the same element is exactly alike… NOT SO. They do have the same # of protons; however, they do not necessarily have the same # of neutrons! • This means that different atoms of the same element may/will have different masses. • These are: isotopes (atoms having the ...
Lecture 5 (2.1-2.3)
... 2.3 Dalton’s Atomic Theory • Postulates of Dalton’s atomic theory (1808) 1. Matter consist of small, indivisible and indestructible atoms. 2. All atoms of an element are identical in mass and different from the atoms of other elements. 3. Compounds result from chemical combinations of different ele ...
... 2.3 Dalton’s Atomic Theory • Postulates of Dalton’s atomic theory (1808) 1. Matter consist of small, indivisible and indestructible atoms. 2. All atoms of an element are identical in mass and different from the atoms of other elements. 3. Compounds result from chemical combinations of different ele ...
Atoms and atomic spectra
... Tiny nucleus with protons and neutrons (~99.98% of mass) Surrounded by large diffuse cloud of low mass electrons ...
... Tiny nucleus with protons and neutrons (~99.98% of mass) Surrounded by large diffuse cloud of low mass electrons ...