2.1 PowerPoint
... Atoms are indestructible and cannot be divided into smaller particles(Atoms are indivisible) All atoms of one element are exactly alike, but they are different from atoms of other elements. ...
... Atoms are indestructible and cannot be divided into smaller particles(Atoms are indivisible) All atoms of one element are exactly alike, but they are different from atoms of other elements. ...
Review Worksheet
... 1. What is the electron configuration of cadmium (Cd)? 2. What is the noble gas (shorthand) electron configuration of copper (Cu)? 3. What group on the periodic table comprises the alkali metals? 4. What do elements in the same group have in common with one another? 5. Why does electronegativity dec ...
... 1. What is the electron configuration of cadmium (Cd)? 2. What is the noble gas (shorthand) electron configuration of copper (Cu)? 3. What group on the periodic table comprises the alkali metals? 4. What do elements in the same group have in common with one another? 5. Why does electronegativity dec ...
Defining the Atom - World of Teaching
... • The modern atomic thought began with John Dalton (1766-1844) • Dalton used experimental method to transform Democritus’s idea of atoms into a scientific theory. • Dalton studied the ratios in which elements combine and the result was Dalton’s atomic theory. ...
... • The modern atomic thought began with John Dalton (1766-1844) • Dalton used experimental method to transform Democritus’s idea of atoms into a scientific theory. • Dalton studied the ratios in which elements combine and the result was Dalton’s atomic theory. ...
Topic 2
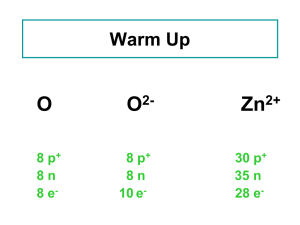
... negatively charged ion, called an anion (more electrons than protons). i.e, Cl– An atom that loses electrons becomes a positively charged ion, called a cation (more protons than electrons). i.e., Na+ – An ionic compound is a compound composed of cations and anions. Answer the following questions for ...
... negatively charged ion, called an anion (more electrons than protons). i.e, Cl– An atom that loses electrons becomes a positively charged ion, called a cation (more protons than electrons). i.e., Na+ – An ionic compound is a compound composed of cations and anions. Answer the following questions for ...
Isotopes-Chemistry
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
H - JMap
... 56 As the volume of a fixed mass of a gas increases at constant temperature, the pressure of the gas ...
... 56 As the volume of a fixed mass of a gas increases at constant temperature, the pressure of the gas ...
Chemical Reactions - Waukee Community School District Blogs
... 2. These elements need a subscript 2 after them if ...
... 2. These elements need a subscript 2 after them if ...
Atomic Structure
... • Fusion would be MUCH SAFER than fission • Products are “clean” – not radioactive • Accidents are much less likely and less dangerous (it can’t meltdown) ...
... • Fusion would be MUCH SAFER than fission • Products are “clean” – not radioactive • Accidents are much less likely and less dangerous (it can’t meltdown) ...
HW 2 Key
... a. The vast majority of the atom’s volume is occupied only by the light electrons; electrons are too light to deflect heavy α-particles. This volume comes to be known as the electron cloud. b. The few α-particles that hit the heavy protons concentrated in the very small nucleus were deflected away f ...
... a. The vast majority of the atom’s volume is occupied only by the light electrons; electrons are too light to deflect heavy α-particles. This volume comes to be known as the electron cloud. b. The few α-particles that hit the heavy protons concentrated in the very small nucleus were deflected away f ...
J.E. Strong, Jr. 1/21/2012 How big is a Hydrogen Atom? It is very
... reference. Let’s look at how large a simple hydrogen atom is by comparing it to something more familiar. What is hydrogen (and why do we care)? Hydrogen is the simplest chemical element, with an atomic number of 1, meaning its nucleus consists of a single proton. In its normal state (a gas), a hydro ...
... reference. Let’s look at how large a simple hydrogen atom is by comparing it to something more familiar. What is hydrogen (and why do we care)? Hydrogen is the simplest chemical element, with an atomic number of 1, meaning its nucleus consists of a single proton. In its normal state (a gas), a hydro ...
Chem 110 Exam I Fall 2003
... The balanced chemical equation for the reaction between hydrochloric acid and iron(III) oxide is 6 HCl(aq) + Fe2O3(s) = 3 H2O(l) + 2 FeCl3(aq) We can interpret this to mean that ...
... The balanced chemical equation for the reaction between hydrochloric acid and iron(III) oxide is 6 HCl(aq) + Fe2O3(s) = 3 H2O(l) + 2 FeCl3(aq) We can interpret this to mean that ...
Chemistry to Remember
... often the same, but in certain cases, they might be different. To simplify, references to specific gravity in this text will consider t1 and t2 to be the same (20˚C); therefore, specific gravity compares the density of one substance to the density of water at the same temperature. The specific gravi ...
... often the same, but in certain cases, they might be different. To simplify, references to specific gravity in this text will consider t1 and t2 to be the same (20˚C); therefore, specific gravity compares the density of one substance to the density of water at the same temperature. The specific gravi ...
Matching - hrsbstaff.ednet.ns.ca
... a. Protons, electrons, and neutrons are evenly distributed throughout the volume of the atom. b. The nucleus is made of protons, electrons, and neutrons. c. Electrons are distributed around the nucleus and occupy almost all the volume of the atom. d. The nucleus is made of electrons and protons. ___ ...
... a. Protons, electrons, and neutrons are evenly distributed throughout the volume of the atom. b. The nucleus is made of protons, electrons, and neutrons. c. Electrons are distributed around the nucleus and occupy almost all the volume of the atom. d. The nucleus is made of electrons and protons. ___ ...
Atomic Theory
... What’s Wrong with Dalton’s Theory? Problem with Dalton’s atomic theory- atoms can ...
... What’s Wrong with Dalton’s Theory? Problem with Dalton’s atomic theory- atoms can ...
Investigating Atoms and Atomic Theory
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
Investigating Atoms and Atomic Theory
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
Name Honors Chemistry ____/____/____ History of the Atom
... 18. ____________________ Who claimed that atoms of the same element have the same physical and chemical properties? 19. ____________________ Who was able to deduce the relationship between energy and frequency of radiation? 20. ____________________ Which theorist would NOT have been considered an at ...
... 18. ____________________ Who claimed that atoms of the same element have the same physical and chemical properties? 19. ____________________ Who was able to deduce the relationship between energy and frequency of radiation? 20. ____________________ Which theorist would NOT have been considered an at ...
Investigating Atoms and Atomic Theory
... This could only mean that the gold atoms in the sheet were mostly open space. Atoms were not a pudding filled with a positively charged material. Rutherford concluded that an atom had a small, dense, positively charged center that repelled his positively charged “bullets.” He called the center ...
... This could only mean that the gold atoms in the sheet were mostly open space. Atoms were not a pudding filled with a positively charged material. Rutherford concluded that an atom had a small, dense, positively charged center that repelled his positively charged “bullets.” He called the center ...
Answer Key - La Quinta High School
... 1. All chemical reactions do produce some evidence that the reaction has occurred, but sometimes this evidence may not be visual and may not be very obvious. For example, when very dilute aqueous solutions of acids and bases are mixed, the neutralization reaction H+(aq) + OH–(aq) ! H2O(l) takes plac ...
... 1. All chemical reactions do produce some evidence that the reaction has occurred, but sometimes this evidence may not be visual and may not be very obvious. For example, when very dilute aqueous solutions of acids and bases are mixed, the neutralization reaction H+(aq) + OH–(aq) ! H2O(l) takes plac ...
Atomic emission spectrum
... This line spectrum is also called the Atomic Spectrum because it originates in the element. Each element has a different atomic spectrum.The production of line spectra by the atoms of an element, indicates that an atom can radiate only certain amount of energy. This leads to the conclusion that e ...
... This line spectrum is also called the Atomic Spectrum because it originates in the element. Each element has a different atomic spectrum.The production of line spectra by the atoms of an element, indicates that an atom can radiate only certain amount of energy. This leads to the conclusion that e ...