Section 6.1: Covalent Bonding Basics
... atoms that are not the same. A nonpolar covalent bond is a covalent bond in which the bonding electrons are equally attracted to both bonded atoms. A polar covalent bond is a covalent bond in which a shared pair of electrons is held more closely by one of the atoms. The ability of an atom to attrac ...
... atoms that are not the same. A nonpolar covalent bond is a covalent bond in which the bonding electrons are equally attracted to both bonded atoms. A polar covalent bond is a covalent bond in which a shared pair of electrons is held more closely by one of the atoms. The ability of an atom to attrac ...
1) Basic familiarity with Atomic Labels. You will need a Periodic
... 5x10-3 mol l-1). The average volume of blood in a human male is a 4.7 litres. What mass of cholesterol does the blood of a healthy human male contain? According to US Department of Agriculture figures, a tablespoon of butter (14g) contains 30 mg of cholesterol. How many moles of cholesterol are in a ...
... 5x10-3 mol l-1). The average volume of blood in a human male is a 4.7 litres. What mass of cholesterol does the blood of a healthy human male contain? According to US Department of Agriculture figures, a tablespoon of butter (14g) contains 30 mg of cholesterol. How many moles of cholesterol are in a ...
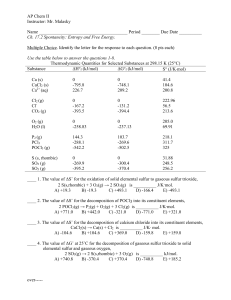
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
Ch 3 Jeopardy Review Game
... Answers for Potpourri 200 1. It is impossible to predict a specific location and momentum (energy) for an electron. (Heisenberg Uncertainty Principle) 2. The math does not correspond to data for any atom other than H (one electron) ...
... Answers for Potpourri 200 1. It is impossible to predict a specific location and momentum (energy) for an electron. (Heisenberg Uncertainty Principle) 2. The math does not correspond to data for any atom other than H (one electron) ...
AP Thermo I Notes
... First Law of Thermodynamics section 5.2 Energy(E) is neither created nor destroyed. The internal energy of a system is the sum of ...
... First Law of Thermodynamics section 5.2 Energy(E) is neither created nor destroyed. The internal energy of a system is the sum of ...
2005 - NESACS
... 42. The hydrogen emission spectrum for galaxy NGC 3310 is shown below. Marked on the spectrum with a vertical line is the red hydrogen emission line, Hα, at 6562.8 Å (656.2 nm) that originates from the Balmer series (32) at the spot where it would be found in a hydrogen spectrum produced in a labor ...
... 42. The hydrogen emission spectrum for galaxy NGC 3310 is shown below. Marked on the spectrum with a vertical line is the red hydrogen emission line, Hα, at 6562.8 Å (656.2 nm) that originates from the Balmer series (32) at the spot where it would be found in a hydrogen spectrum produced in a labor ...
20161010170338
... mass, and atoms of different elements have different masses. 3. Compounds contain atoms of more than one element. 4. In a particular compound, atoms of different elements always combine in the same way ...
... mass, and atoms of different elements have different masses. 3. Compounds contain atoms of more than one element. 4. In a particular compound, atoms of different elements always combine in the same way ...
Atoms and Elements Atoms and Elements
... alpha - a helium cation - a beta - supercharged electrons - b gamma - high energy emission - g Note that a and b are massive and charged, but g radiation has no charge or mass MAR ...
... alpha - a helium cation - a beta - supercharged electrons - b gamma - high energy emission - g Note that a and b are massive and charged, but g radiation has no charge or mass MAR ...
Chapter 12 Oxidation-Reduction Reactions
... The operational definition of oxidation is any reaction involving molecular oxygen. The theoretical definition of oxidation is the loss of electrons. The operational definition of reduction is the extracting of metals from metal ore. The theoretical definition of reduction is the gain of electrons. ...
... The operational definition of oxidation is any reaction involving molecular oxygen. The theoretical definition of oxidation is the loss of electrons. The operational definition of reduction is the extracting of metals from metal ore. The theoretical definition of reduction is the gain of electrons. ...
3_Atomic Spectrum-Bohr
... The notion of negative electrons orbiting a positively charged nucleus does not work, since an accelerating charge would emit continuously, lose energy and fall into the nucleus. This theory only worked for hydrogen, and for no other element. The spectra of larger atoms are considerably more complic ...
... The notion of negative electrons orbiting a positively charged nucleus does not work, since an accelerating charge would emit continuously, lose energy and fall into the nucleus. This theory only worked for hydrogen, and for no other element. The spectra of larger atoms are considerably more complic ...
makeup6
... (D) In general the lattice energy increases as the charge on the anion and cation increase and increases as the size of the anion and cation decrease. 13. The pH of a saturated solution of calcium hydroxide is 12.40. What is Ksp for this salt? (A) 6.3 x 10¯38 (B) 1.58 x 10¯25 (C) 7.9 x 10¯6 (D) 1.58 ...
... (D) In general the lattice energy increases as the charge on the anion and cation increase and increases as the size of the anion and cation decrease. 13. The pH of a saturated solution of calcium hydroxide is 12.40. What is Ksp for this salt? (A) 6.3 x 10¯38 (B) 1.58 x 10¯25 (C) 7.9 x 10¯6 (D) 1.58 ...
Ch. 5.1 PPT - Warren County Schools
... In the quantum mechanical model, the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The cloud is more dense where the probability of finding the electron is high. ...
... In the quantum mechanical model, the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The cloud is more dense where the probability of finding the electron is high. ...
Chemistry I Exams and Keys Corrected 2016 Season
... element that combine with a fixed weight of the other are in a ratio of small whole numbers. B) The rate of any chemical reaction is proportional to the product of the masses of the reacting substances, with each mass raised to a power equal to its coefficient. C) During any chemical reaction, nucle ...
... element that combine with a fixed weight of the other are in a ratio of small whole numbers. B) The rate of any chemical reaction is proportional to the product of the masses of the reacting substances, with each mass raised to a power equal to its coefficient. C) During any chemical reaction, nucle ...
Chapter 3 Lecture Notes
... 1 mole of something = 6.02 x 1023 of that thing. • This number is called Avogadro’s number. • Thus, 1 mole of carbon atoms = 6.02 x 1023 carbon atoms. ...
... 1 mole of something = 6.02 x 1023 of that thing. • This number is called Avogadro’s number. • Thus, 1 mole of carbon atoms = 6.02 x 1023 carbon atoms. ...
Spectra and Atomic Structure
... component frequencies • Continuous spectrum is emitted by solid, liquid, and dense gas • Hot gas has characteristic emission spectrum • Continuous spectrum incident on cool, thin gas gives characteristic absorption spectrum ...
... component frequencies • Continuous spectrum is emitted by solid, liquid, and dense gas • Hot gas has characteristic emission spectrum • Continuous spectrum incident on cool, thin gas gives characteristic absorption spectrum ...
Atomic Structure
... Write complete and shorthand electron configurations as well as orbital diagrams for an atom or ion of an element. Identify the number and location of the valence electrons in an atom. Apply the trends in atomic properties such as atomic radii, ionization energy, electronegativity, electron affin ...
... Write complete and shorthand electron configurations as well as orbital diagrams for an atom or ion of an element. Identify the number and location of the valence electrons in an atom. Apply the trends in atomic properties such as atomic radii, ionization energy, electronegativity, electron affin ...
Lecture 6 – Thermochemistry
... Two equal mass samples of water produced by: T 1. Heating one from 20°C to 50°C. 2. Cooling the other from 100°C to 50°C. have identical final H (and V, P, E…). ...
... Two equal mass samples of water produced by: T 1. Heating one from 20°C to 50°C. 2. Cooling the other from 100°C to 50°C. have identical final H (and V, P, E…). ...
DEFINING THE ATOM - BradyMathScience
... ________ 14. The number of neutrons in the nucleus of an atom can be calculated by a. adding together the numbers of electrons and protons. b. subtracting the number of protons from the number of electrons. c. subtracting the number of protons from the mass number. d. adding the mass number to the n ...
... ________ 14. The number of neutrons in the nucleus of an atom can be calculated by a. adding together the numbers of electrons and protons. b. subtracting the number of protons from the number of electrons. c. subtracting the number of protons from the mass number. d. adding the mass number to the n ...
Chemistry - Onslow College
... Writing word equations and balanced chemical equations for inorganic reactions By the end of this topic students will be able to 1. use solubility rules to predict precipitation and identify the precipitate. 2. carry out precipitation reactions and report experimental observations 3. from experime ...
... Writing word equations and balanced chemical equations for inorganic reactions By the end of this topic students will be able to 1. use solubility rules to predict precipitation and identify the precipitate. 2. carry out precipitation reactions and report experimental observations 3. from experime ...
Name_______________________________________________
... d. the more likely it is that the substances is polar-covalent 2. In general, intermolecular forces are a. stronger than bonds that join atoms in molecules b. weaker than bonds that join atoms in molecules, but stronger than ionic bonds c. stronger than bonds that join metal atoms in solid metals d. ...
... d. the more likely it is that the substances is polar-covalent 2. In general, intermolecular forces are a. stronger than bonds that join atoms in molecules b. weaker than bonds that join atoms in molecules, but stronger than ionic bonds c. stronger than bonds that join metal atoms in solid metals d. ...
ATOMIC STRUCTURE
... (1852–1908), a French physicist, was studying fluorescence by wrapping photographic film in black paper, placing a few crystals of the fluorescing chemical on top of the paper, and then placing the package in strong sunlight. If the glow was like ordinary light, it would not pass through the paper. ...
... (1852–1908), a French physicist, was studying fluorescence by wrapping photographic film in black paper, placing a few crystals of the fluorescing chemical on top of the paper, and then placing the package in strong sunlight. If the glow was like ordinary light, it would not pass through the paper. ...