Atomic History Timeline Grading Rubric
... • Electrons travel in specified energy levels • Spectrum lines produced when electrons move • Electrons have properties of both waves and particles • Group of waves named after scientist • “uncertainty principle” • Impossible to determine the position and the momentum of a particle at the same time ...
... • Electrons travel in specified energy levels • Spectrum lines produced when electrons move • Electrons have properties of both waves and particles • Group of waves named after scientist • “uncertainty principle” • Impossible to determine the position and the momentum of a particle at the same time ...
AP* Chemistry ELECTROCHEMISTRY Terms to Know
... want to get picky and eliminate the error introduced by resistance, you attach a variable-external power source called a potentiometer. Adjust it so that zero current flows—the accurate voltage is then equal in magnitude but opposite in sign to the reading on the potentiometer. ORIGIN OF STANDARD RE ...
... want to get picky and eliminate the error introduced by resistance, you attach a variable-external power source called a potentiometer. Adjust it so that zero current flows—the accurate voltage is then equal in magnitude but opposite in sign to the reading on the potentiometer. ORIGIN OF STANDARD RE ...
Microbial Biogeochemistry
... Bacteria that are able to use the most energetic reactions in their surrounding environment will dominate that microenvironment. Transport combined with the microbial sources and sinks will determine the resulting chemical gradients. Chemical gradients can be transient as substrates are exhausted or ...
... Bacteria that are able to use the most energetic reactions in their surrounding environment will dominate that microenvironment. Transport combined with the microbial sources and sinks will determine the resulting chemical gradients. Chemical gradients can be transient as substrates are exhausted or ...
Class IX Chapter 4 – Structure of the Atom Science
... If the α-scattering experiment is carried out using a foil of a metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from oth ...
... If the α-scattering experiment is carried out using a foil of a metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from oth ...
+2 - Fort Thomas Independent Schools
... • Oxidation numbers are numbers assigned to the atoms in a molecular compound or ion that indicates the general distribution of electrons among bonded atoms. • Oxidation numbers are not actual charges. • Oxidation numbers can be useful in naming compounds and writing formulas. ...
... • Oxidation numbers are numbers assigned to the atoms in a molecular compound or ion that indicates the general distribution of electrons among bonded atoms. • Oxidation numbers are not actual charges. • Oxidation numbers can be useful in naming compounds and writing formulas. ...
atoms
... Dalton’s Atomic Theory (1808) Each element is made up of tiny particles called atoms. The atoms of a given element are identical; the atoms of different elements are different in some fundamental way or ...
... Dalton’s Atomic Theory (1808) Each element is made up of tiny particles called atoms. The atoms of a given element are identical; the atoms of different elements are different in some fundamental way or ...
2013 us national chemistry olympiad
... 5. [12] Write net equations for each of the reactions below. Use ionic and molecular formulas as appropriate and omit formulas for all ions or molecules that do not take part in a reaction. Write structural formulas for all organic substances. You need not balance the equations or specify physical ...
... 5. [12] Write net equations for each of the reactions below. Use ionic and molecular formulas as appropriate and omit formulas for all ions or molecules that do not take part in a reaction. Write structural formulas for all organic substances. You need not balance the equations or specify physical ...
Elements compounds and mixtures
... i.e. it has a fix boiling point. An impure liquid could boil higher than the expected boiling point and over a range of temperature. – A pure substance melts quite sharply at the melting point. An impure solid melts below its expected melting point and more slowly over a wider temperature range. ...
... i.e. it has a fix boiling point. An impure liquid could boil higher than the expected boiling point and over a range of temperature. – A pure substance melts quite sharply at the melting point. An impure solid melts below its expected melting point and more slowly over a wider temperature range. ...
SIA Chapter 12 Atoms PP
... emitted by elements when they are made to glow— identifies each element by its characteristic pattern • Each element emits a distinctive glow when energized and displays a ...
... emitted by elements when they are made to glow— identifies each element by its characteristic pattern • Each element emits a distinctive glow when energized and displays a ...
4.3 Nuclear Energy
... lose almost all its KE to the moderator atom. – The moderator atoms should not absorb neutrons but should scatter them instead. – In practice, heavy water (D2O i.e. 2H2O) is chosen as the moderator. (Heavy water is different from hard water) 19.1 The atomic model ...
... lose almost all its KE to the moderator atom. – The moderator atoms should not absorb neutrons but should scatter them instead. – In practice, heavy water (D2O i.e. 2H2O) is chosen as the moderator. (Heavy water is different from hard water) 19.1 The atomic model ...
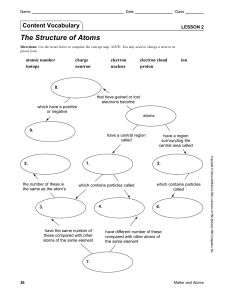
Lesson 2 | The Structure of Atoms
... 2. Atoms are composed of several basic types of very small particles; the number of each of these particles gives the different kinds of atoms their unique identity. 3. The region at the center of an atom that contains most of the mass of the atom is called the nucleus. a. A positively charged parti ...
... 2. Atoms are composed of several basic types of very small particles; the number of each of these particles gives the different kinds of atoms their unique identity. 3. The region at the center of an atom that contains most of the mass of the atom is called the nucleus. a. A positively charged parti ...
The Elements of Group 15 (5A, V, VA) The Nitrogen Group
... Phosphorus is a tetrameric solid (white phosphorus) in its standard state (P4(s)), although it exists as many allotropes. White phosphorus reacts with oxygen (combusts), so must be stored under water. Formerly used in matches. ...
... Phosphorus is a tetrameric solid (white phosphorus) in its standard state (P4(s)), although it exists as many allotropes. White phosphorus reacts with oxygen (combusts), so must be stored under water. Formerly used in matches. ...
3 - Greene County ESC
... number of protons may or may not have the same mass. Thos with different masses (different numbers of neutrons) are called isotopes. 2. Illustrate that atoms with the same number of positively charged protons and negatively charged electrons are electrically neutral. 4. Show that when elements are l ...
... number of protons may or may not have the same mass. Thos with different masses (different numbers of neutrons) are called isotopes. 2. Illustrate that atoms with the same number of positively charged protons and negatively charged electrons are electrically neutral. 4. Show that when elements are l ...
CHAPTER 4 | Solution Chemistry and the Hydrosphere
... If Cr3+ and Cd2+ can be removed from wastewater by treatment with NaOH(aq), they must form insoluble salts and, therefore, can be filtered off. Analyze Referring to Table 4.5 we see that all hydroxides, except those of Na + (and other alkali metals and NH4+) and those of Ba2+, Ca2+, Sr2+, are insolu ...
... If Cr3+ and Cd2+ can be removed from wastewater by treatment with NaOH(aq), they must form insoluble salts and, therefore, can be filtered off. Analyze Referring to Table 4.5 we see that all hydroxides, except those of Na + (and other alkali metals and NH4+) and those of Ba2+, Ca2+, Sr2+, are insolu ...
IB 1 CHEMISTRY
... mass, far too small to weigh. Ex. Calculate the mass of one hydrogen-1 atom. 1,67 ▪ 10-27 kg (+ 9,1 10-31 kg) = 0,00000000000000000000000167 g ...
... mass, far too small to weigh. Ex. Calculate the mass of one hydrogen-1 atom. 1,67 ▪ 10-27 kg (+ 9,1 10-31 kg) = 0,00000000000000000000000167 g ...
Conservation of Energy in chemical reactions, Hess`s Law
... What kind of change is occurring this time, and what elements are involved? What is the critical difference? What would H be in the second reaction? _____ Why does this make sense? ...
... What kind of change is occurring this time, and what elements are involved? What is the critical difference? What would H be in the second reaction? _____ Why does this make sense? ...
ATOMS
... – Nucleus: the center of the atom that contains the mass of the atom – Electron Cloud : the region that surrounds the nucleus that contains most of the space in the atom ...
... – Nucleus: the center of the atom that contains the mass of the atom – Electron Cloud : the region that surrounds the nucleus that contains most of the space in the atom ...
Document
... electrons are assumed to revolve around the atomic nucleus in discrete orbitals, and the position of any particular electron is more or less well defined in terms of its orbital. Electrons are permitted to have only specific values of energy. ...
... electrons are assumed to revolve around the atomic nucleus in discrete orbitals, and the position of any particular electron is more or less well defined in terms of its orbital. Electrons are permitted to have only specific values of energy. ...
PPT of Notes
... nuclides or as percentages of nuclides) A “rough estimate” may be obtained if the mass numbers are utilized ...
... nuclides or as percentages of nuclides) A “rough estimate” may be obtained if the mass numbers are utilized ...
File
... Thus there are two effects of increasing temperature: greater collision intensity and more frequent collisions. Activation Energy -minimum energy needed for a reaction to take place. A higher temp, a greater fraction of the molecules have KE > = the Ea. So this just says to have a reaction you need ...
... Thus there are two effects of increasing temperature: greater collision intensity and more frequent collisions. Activation Energy -minimum energy needed for a reaction to take place. A higher temp, a greater fraction of the molecules have KE > = the Ea. So this just says to have a reaction you need ...
(1/V m C) +
... The primary process of light absorption in photochemical reaction is independent of temp. Effect of temp depends up on the type and nature of secondary process. If the secondary process involves the active atom or radical produced in the primary process, its activation energy is very small and thus ...
... The primary process of light absorption in photochemical reaction is independent of temp. Effect of temp depends up on the type and nature of secondary process. If the secondary process involves the active atom or radical produced in the primary process, its activation energy is very small and thus ...
2002 local exam - Virginia Section
... The answers for questions 4 through 7 follow. Select the lettered choice that best fits the statement for each question and fill in the corresponding block on the answer sheet. You may use a choice more than once, once, or not at all. (A) density (B) equilibrium constant (C) freezing point (D) molar ...
... The answers for questions 4 through 7 follow. Select the lettered choice that best fits the statement for each question and fill in the corresponding block on the answer sheet. You may use a choice more than once, once, or not at all. (A) density (B) equilibrium constant (C) freezing point (D) molar ...
Practice Exam-Final Fall 2016 W-Ans
... allowed? Hint: l can have only 0, 1, 2, 3, ……(n-1), but no negative numbers. ...
... allowed? Hint: l can have only 0, 1, 2, 3, ……(n-1), but no negative numbers. ...