Atomic Structure
... a) Three isotopes of sulfur are sulfur-32, sulfur33, and sulfur-34. Write the complete symbol for each isotope, including the atomic number and the mass number. b) How many neutrons, protons, and electrons are in Na+ with a mass number of 24? What is its atomic number? ...
... a) Three isotopes of sulfur are sulfur-32, sulfur33, and sulfur-34. Write the complete symbol for each isotope, including the atomic number and the mass number. b) How many neutrons, protons, and electrons are in Na+ with a mass number of 24? What is its atomic number? ...
Notebook LAyout for Atoms Unit- Page 46+
... Mrs. Aguirre’s Webpage: http://www.quia.com/profiles/caguirre ...
... Mrs. Aguirre’s Webpage: http://www.quia.com/profiles/caguirre ...
TIPS for NET-IONIC EQUATIONS A.P. Chemistry (long form)
... 1. dilute sulfuric acid is added to a solution of barium acetate 2. solutions of sodium phosphate and calcium chloride are mixed 3. hydrogen sulfide gas is bubbled through a solution of silver nitrate 4. manganese(II) nitrate solution is mixed with sodium hydroxide solution 5. solutions of zinc sulf ...
... 1. dilute sulfuric acid is added to a solution of barium acetate 2. solutions of sodium phosphate and calcium chloride are mixed 3. hydrogen sulfide gas is bubbled through a solution of silver nitrate 4. manganese(II) nitrate solution is mixed with sodium hydroxide solution 5. solutions of zinc sulf ...
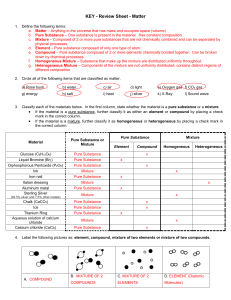
Matter Test Review Sheet
... e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly d ...
... e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly d ...
CHEMISTRY SAMPLE PAPER - I
... (b) the reduction of Cr2O3 with AI is thermodynamically feasible, yet it does not occur at room temperature. (c) pine oil is used in froth floatation method. 3 23. Explain the following facts (a) transition metals act as catalysts. (b) chromium group elements have the highest melting points in their ...
... (b) the reduction of Cr2O3 with AI is thermodynamically feasible, yet it does not occur at room temperature. (c) pine oil is used in froth floatation method. 3 23. Explain the following facts (a) transition metals act as catalysts. (b) chromium group elements have the highest melting points in their ...
Chapter 24. Organic Chemistry
... An ability of an atom to attract toward itself the electron cloud in a chemical bond Electronegativity is a relative concept, meaning that an electronegativilty of one atom can be measured relative to another atom Generally electronegativity increases from left to right acros a period in the periodi ...
... An ability of an atom to attract toward itself the electron cloud in a chemical bond Electronegativity is a relative concept, meaning that an electronegativilty of one atom can be measured relative to another atom Generally electronegativity increases from left to right acros a period in the periodi ...
AP Chemistry Summer Assignment 2016
... Predict whether the following combinations will result in a reaction. Write a balanced reaction for those reactions. Indicate you understand the specific reactions by writing the net ionic equation for the reaction. Hopefully you would have memorized the solubility rules before attempting to answer ...
... Predict whether the following combinations will result in a reaction. Write a balanced reaction for those reactions. Indicate you understand the specific reactions by writing the net ionic equation for the reaction. Hopefully you would have memorized the solubility rules before attempting to answer ...
Practice Test Stoichiometry
... 42.) Which of the following statements is always true concerning a reaction represented by the following balanced chemical equation? 2C2H6(g) + 7O2(g) 6H2O(l) + 4CO2(g) A) If we have equal masses of C2H6 and O2, there is no limiting reactant. B) If we have an equal number of moles of C2H6 and O2, ...
... 42.) Which of the following statements is always true concerning a reaction represented by the following balanced chemical equation? 2C2H6(g) + 7O2(g) 6H2O(l) + 4CO2(g) A) If we have equal masses of C2H6 and O2, there is no limiting reactant. B) If we have an equal number of moles of C2H6 and O2, ...
Science SOL CH
... o What factors influence the stability of a nuclide? o What are the different types of nuclear decay? o What happens when an atom undergoes nuclear decay? o Is there a way to predict what type of decay a particular radioisotope will undergo? o How can I use half-life data to calculate the amount of ...
... o What factors influence the stability of a nuclide? o What are the different types of nuclear decay? o What happens when an atom undergoes nuclear decay? o Is there a way to predict what type of decay a particular radioisotope will undergo? o How can I use half-life data to calculate the amount of ...
chemistry intro and lesson 1
... second energy level and eight in the third energy level. We will not study the structure of atoms with more than three levels of energy levels. Each energy level must be filled before electrons occupy the next one. Electrons are so light they are considered to have zero mass. Electrons have a negati ...
... second energy level and eight in the third energy level. We will not study the structure of atoms with more than three levels of energy levels. Each energy level must be filled before electrons occupy the next one. Electrons are so light they are considered to have zero mass. Electrons have a negati ...
chemistry
... word or expression that, of those given, best completes the statement or answers the question. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. 35 Which general trend is found in Period 3 as the elements are considered in order of increasing ato ...
... word or expression that, of those given, best completes the statement or answers the question. Some questions may require the use of the 2011 Edition Reference Tables for Physical Setting/Chemistry. 35 Which general trend is found in Period 3 as the elements are considered in order of increasing ato ...
Slideshow
... In each case, we still have a Hydrogen atom – the atomic number hasn’t changed. The mass number, however, would be different. Option 1: 1 proton + 0 neutrons gives a mass number of 1 Option 2: 1 proton + 1 neutron gives a mass number of 2 Option 3: 1 proton + 2 neutrons gives a mass number of 3 ...
... In each case, we still have a Hydrogen atom – the atomic number hasn’t changed. The mass number, however, would be different. Option 1: 1 proton + 0 neutrons gives a mass number of 1 Option 2: 1 proton + 1 neutron gives a mass number of 2 Option 3: 1 proton + 2 neutrons gives a mass number of 3 ...
Contents
... Almost everything we utilize today has been produced by means of a chemical reaction. For example, the shells of electronic devices are usually made of plastic. The plastic was synthesized from a chemical compound which itself had been produced from oil. The silicon at the heart of most electronic d ...
... Almost everything we utilize today has been produced by means of a chemical reaction. For example, the shells of electronic devices are usually made of plastic. The plastic was synthesized from a chemical compound which itself had been produced from oil. The silicon at the heart of most electronic d ...
Unit 6 Slides
... ■ LO 1.7 The student is able to describe the electron structure of the atom, using PES (photoelectron spectroscopy) data, ionization energy data, and/or Coulomb’s Law to construct explanations of how the energies of electrons within shells in atoms vary. ■ LO 1.9 The student is able to predict and/o ...
... ■ LO 1.7 The student is able to describe the electron structure of the atom, using PES (photoelectron spectroscopy) data, ionization energy data, and/or Coulomb’s Law to construct explanations of how the energies of electrons within shells in atoms vary. ■ LO 1.9 The student is able to predict and/o ...
sci_tech_electricity01_pos_neg
... The word electricity, comes from the Greek word “elektron”, which was Greek for amber. As early as 600 B.C. the Greeks realized that amber, a fossilized resin from tree sap, would pick up small bits of dust, lint and other light materials when it was rubbed. What the Greeks discovered was static ele ...
... The word electricity, comes from the Greek word “elektron”, which was Greek for amber. As early as 600 B.C. the Greeks realized that amber, a fossilized resin from tree sap, would pick up small bits of dust, lint and other light materials when it was rubbed. What the Greeks discovered was static ele ...
chemistry
... Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the last page of this examination booklet. Turn to the last page and fold it along the ...
... Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the last page of this examination booklet. Turn to the last page and fold it along the ...
Chem G 9
... Students should appreciate that at the time that Dalton’s theory was proposed nothing was known about the internal structure of the atom. As a result of our increasing knowledge about atomic structure we now know that statement #2 and statement #3 are no longer true. Students should attempt to modif ...
... Students should appreciate that at the time that Dalton’s theory was proposed nothing was known about the internal structure of the atom. As a result of our increasing knowledge about atomic structure we now know that statement #2 and statement #3 are no longer true. Students should attempt to modif ...
Bellringer Chapter 11 Objectives Chapter 11 The Beginning of the
... • Dalton based his theory on observations of how elements combine. • Thomson discovered electrons in atoms. • Rutherford discovered that atoms are mostly empty space with a dense, positive nucleus. • Bohr proposed that electrons are located in levels at certain distances from the nucleus. • The elec ...
... • Dalton based his theory on observations of how elements combine. • Thomson discovered electrons in atoms. • Rutherford discovered that atoms are mostly empty space with a dense, positive nucleus. • Bohr proposed that electrons are located in levels at certain distances from the nucleus. • The elec ...
chapter4
... • Some of these particles are called protons – charge = +1 – mass is about the same as a hydrogen atom • Since protons and electrons have the same amount of charge, for the atom to be neutral there must be equal numbers of protons and electrons • The other particle is called a neutron – has no charg ...
... • Some of these particles are called protons – charge = +1 – mass is about the same as a hydrogen atom • Since protons and electrons have the same amount of charge, for the atom to be neutral there must be equal numbers of protons and electrons • The other particle is called a neutron – has no charg ...
LESSON PLAN School : State Senior High School ……………… The
... This surprised Thompson, because the atoms of the gas were uncharged. Where had the negative charged come from? Thompson conclude that the negative charges came from within the atom). Since the gas was known to be neutral, having no charge, he reasoned that there must be positively charged particle ...
... This surprised Thompson, because the atoms of the gas were uncharged. Where had the negative charged come from? Thompson conclude that the negative charges came from within the atom). Since the gas was known to be neutral, having no charge, he reasoned that there must be positively charged particle ...
Introduction to Computational Chemistry
... • Semiempirical methods rely on parametrization of some of the integrals that occur in the solution of the Schrödinger equation using experimental data. • Density functional methods are based on the specification of a certain functional form for the electron density in the molecule. B. Utility and A ...
... • Semiempirical methods rely on parametrization of some of the integrals that occur in the solution of the Schrödinger equation using experimental data. • Density functional methods are based on the specification of a certain functional form for the electron density in the molecule. B. Utility and A ...
Chapter 3 Notes
... that contains as many atoms, molecules, ions, or other elementary units as the number of atoms in 12.01 g C. The number is 6.02 × 1023, or Avogadro's number. ...
... that contains as many atoms, molecules, ions, or other elementary units as the number of atoms in 12.01 g C. The number is 6.02 × 1023, or Avogadro's number. ...
Document
... Electron Spin (Uhlenbeck and Goudsmit, 1925) Pauli postulated in 1925 that an electron can exist in two distinct states and introduced in a rather ad hoc manner a fourth quantum number to describe the two states. Although with this he could explain the Stern-Gerlach experiment, no interpretation was ...
... Electron Spin (Uhlenbeck and Goudsmit, 1925) Pauli postulated in 1925 that an electron can exist in two distinct states and introduced in a rather ad hoc manner a fourth quantum number to describe the two states. Although with this he could explain the Stern-Gerlach experiment, no interpretation was ...
How many grams of oxygen are made if 3.75 moles of KClO 3
... What phase changes release energy? and what phase changes require energy? Explain why freezing is an exothermic change of state. Describe the relationship between temperature and pressure. Describe conduction in terms of molecules bumping into each other to transfer energy. Explain why there is bett ...
... What phase changes release energy? and what phase changes require energy? Explain why freezing is an exothermic change of state. Describe the relationship between temperature and pressure. Describe conduction in terms of molecules bumping into each other to transfer energy. Explain why there is bett ...