Periodic Trends
... Question What trends can you find in the periodic table? Trend: a general or prevailing tendency. Every element does not need to follow the trend. Often the exceptions are as enlightening as the trend. The trends in a periodic table of the elements depend on the arrangement of elements in that table ...
... Question What trends can you find in the periodic table? Trend: a general or prevailing tendency. Every element does not need to follow the trend. Often the exceptions are as enlightening as the trend. The trends in a periodic table of the elements depend on the arrangement of elements in that table ...
Science Questions
... Reason: Any metal will combine chemically with any non-metal to form ionic bonds that hold the molecule together. ...
... Reason: Any metal will combine chemically with any non-metal to form ionic bonds that hold the molecule together. ...
Periodic Properties of Elements
... • Number of electrons = (usually) same number as protons so it is neutral ...
... • Number of electrons = (usually) same number as protons so it is neutral ...
Chapter 5 Notes
... The s-block elements consist of the elements in groups 1 and 2 of the periodic table. Electrons from these elements fill the s orbital of each period. Group 1 (ALKALI METALS) fill the s orbital with 1 electron. These elements are considered to be reactive metals because they are not readily found in ...
... The s-block elements consist of the elements in groups 1 and 2 of the periodic table. Electrons from these elements fill the s orbital of each period. Group 1 (ALKALI METALS) fill the s orbital with 1 electron. These elements are considered to be reactive metals because they are not readily found in ...
Atomic Structure and the Periodic Table of Elements: The Secret
... common trends or patterns found within the Periodic Table of Elements. The elements found within the Periodic Table are arranged in a very particular pattern, based on several common similarities or characteristics. In 1869, Dmitri Mendeleev produced a table of elements based on their atomic weights ...
... common trends or patterns found within the Periodic Table of Elements. The elements found within the Periodic Table are arranged in a very particular pattern, based on several common similarities or characteristics. In 1869, Dmitri Mendeleev produced a table of elements based on their atomic weights ...
III. Periodic Trends
... 32. Define Aufbau principle Electrons enter orbitals of lowest energy first “lazy tenant rule” 33. State the Pauli Exclusion Principle. In a particular atom, no two electrons can have the same set of four quantum numbers. If they are in the same orbital they will have opposite spin. 34. State Hunds ...
... 32. Define Aufbau principle Electrons enter orbitals of lowest energy first “lazy tenant rule” 33. State the Pauli Exclusion Principle. In a particular atom, no two electrons can have the same set of four quantum numbers. If they are in the same orbital they will have opposite spin. 34. State Hunds ...
Revising the Periodic Table
... 14. The outermost shell is called the valence shell. 15. Electrons in the valence shell are called valence electrons. 16. Each atom has one or more valence electrons. 17. Most atoms have one or more inner shells of electrons inside the valence shell. 18. The inner shells are part of the atomic core. ...
... 14. The outermost shell is called the valence shell. 15. Electrons in the valence shell are called valence electrons. 16. Each atom has one or more valence electrons. 17. Most atoms have one or more inner shells of electrons inside the valence shell. 18. The inner shells are part of the atomic core. ...
Atomic structure and Periodic table revision guide File
... The boiling points of the noble gases increase with increasing relative atomic mass (going down the group). The elements in Group 1 of the periodic table, known as the alkali metals: are metals with low density (the first three elements in the group are less dense than water) react with non-meta ...
... The boiling points of the noble gases increase with increasing relative atomic mass (going down the group). The elements in Group 1 of the periodic table, known as the alkali metals: are metals with low density (the first three elements in the group are less dense than water) react with non-meta ...
Periodic Table
... Two variable determine the atomic radius of a atom: the number of protons in the nucleus the number of electron energy levels in the atom. The number protons and radius are inversely proportional. As protons increase, the radius decreases. The number of energy levels and radius are proportional. As ...
... Two variable determine the atomic radius of a atom: the number of protons in the nucleus the number of electron energy levels in the atom. The number protons and radius are inversely proportional. As protons increase, the radius decreases. The number of energy levels and radius are proportional. As ...
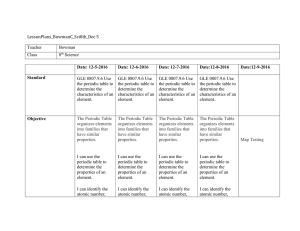
LessonPlans_BowmanC_Sci8th_Dec 5 Teacher Bowman Class 8th
... Objectives of the Lesson Opener: “Repeating Patterns” –New Seating Chart Organize the students in a Pattern: First Letter of their First Name ...
... Objectives of the Lesson Opener: “Repeating Patterns” –New Seating Chart Organize the students in a Pattern: First Letter of their First Name ...
TCSS Physical Science Unit 2 – Atomic Structure Information
... the periodic table. The terms “atomic number” and “atomic mass.” Groups and Periods Song (2:48) – This song is based on the 60’s tune “Happy Together.” This song should help students remember the difference in meaning between Group Numbers and Period Numbers. Reaction (Explosion) of Alkali Metals wi ...
... the periodic table. The terms “atomic number” and “atomic mass.” Groups and Periods Song (2:48) – This song is based on the 60’s tune “Happy Together.” This song should help students remember the difference in meaning between Group Numbers and Period Numbers. Reaction (Explosion) of Alkali Metals wi ...
21 • Electron Transfer Reactions
... Write the quantum numbers for each of the electrons in oxygen, O (Z = 8): ...
... Write the quantum numbers for each of the electrons in oxygen, O (Z = 8): ...
The Periodic Table of the Elements
... • Next came the development of IRON METALLURGY, the extraction of iron from its ores. This led to the start of the Iron Age (3000 years ago). • As humans have learned more about chemistry, metallurgy has expanded to the extraction of many other metals from their ores. ...
... • Next came the development of IRON METALLURGY, the extraction of iron from its ores. This led to the start of the Iron Age (3000 years ago). • As humans have learned more about chemistry, metallurgy has expanded to the extraction of many other metals from their ores. ...
Review guide for Chemistry`s First Semester Exam Unit 1 Thinking
... Describe each of the following lab procedures by indicating what is the main goal for using the procedure, and indicate when you would use them. 1. Decanting Decanting is a technique used for removing excess water from a mixture of solid and liquid or two liquids that do not mix (immiscible). The be ...
... Describe each of the following lab procedures by indicating what is the main goal for using the procedure, and indicate when you would use them. 1. Decanting Decanting is a technique used for removing excess water from a mixture of solid and liquid or two liquids that do not mix (immiscible). The be ...
Periodicity of Elements and Periodic Table CHAPTER – 4
... 1. They are mono atomic. 2. They exist in gaseous state. 3. Outer most shell of these elements is either complete or contains eight electrons. 4. These elements are mostly chemically non-reactive. 5. These elements have no tendency to form compounds (only a few of these compounds are known). Atomic ...
... 1. They are mono atomic. 2. They exist in gaseous state. 3. Outer most shell of these elements is either complete or contains eight electrons. 4. These elements are mostly chemically non-reactive. 5. These elements have no tendency to form compounds (only a few of these compounds are known). Atomic ...
The Periodic Table of the Elements
... persuaded most chemists to accept his periodic table; however, not all elements could be arranged in order of ATOMIC MASS. • In 1911(40 years later) Henry Moseley’s work on atomic spectra led to the discovery that the elements fit the pattern of increasing ATOMIC NUMBER better than atomic mass. ...
... persuaded most chemists to accept his periodic table; however, not all elements could be arranged in order of ATOMIC MASS. • In 1911(40 years later) Henry Moseley’s work on atomic spectra led to the discovery that the elements fit the pattern of increasing ATOMIC NUMBER better than atomic mass. ...
Periodic Table Trends - Magoffin County Schools
... • Another trend that can easily be seen in the periodic table is that of ELECTRONEGATIVITY. • Simply put, Eneg is a measure of an atom’s ability to chemically bond (react) with other atoms. • For Eneg, each atom is assigned a number value from 0 (weakest) to 4 (strongest) . • The closer to 4, the mo ...
... • Another trend that can easily be seen in the periodic table is that of ELECTRONEGATIVITY. • Simply put, Eneg is a measure of an atom’s ability to chemically bond (react) with other atoms. • For Eneg, each atom is assigned a number value from 0 (weakest) to 4 (strongest) . • The closer to 4, the mo ...
REVIEW TEST 4.5 weeks
... neutrons equals? Atomic Mass Number of Protons equals? Atomic Number ...
... neutrons equals? Atomic Mass Number of Protons equals? Atomic Number ...
Ionization energy
... Should equal the arithmetic mean of lithium and potassium. (7+39)/2 = 23, which is the mass of sodium. ...
... Should equal the arithmetic mean of lithium and potassium. (7+39)/2 = 23, which is the mass of sodium. ...
Chemistry - • Elements • Electron Configurations • The Periodic Table
... To predict how many electrons will be in each energy level and sublevel we need to know the energies of electron orbitals. Due to the increasing closeness of the energy levels and the sublevel splitting of the energy level the energy sublevels from one level start to overlap the sublevels of the nex ...
... To predict how many electrons will be in each energy level and sublevel we need to know the energies of electron orbitals. Due to the increasing closeness of the energy levels and the sublevel splitting of the energy level the energy sublevels from one level start to overlap the sublevels of the nex ...
to chapter:3
... Periodic table history History of the periodic table of chemical elements In 1669 German merchant and amateur alchemist Hennig Brand attempted to created a Philosopher’s Stone; an object that supposedly could turn metals into pure gold. He heated residues from boiled urine, and a liquid dropped out ...
... Periodic table history History of the periodic table of chemical elements In 1669 German merchant and amateur alchemist Hennig Brand attempted to created a Philosopher’s Stone; an object that supposedly could turn metals into pure gold. He heated residues from boiled urine, and a liquid dropped out ...
Electron Energy Level
... and nonmetals on the periodic table. They have properties of both metals and nonmetals. Metalloids are more brittle than metals, less brittle than most nonmetallic solids Metalloids are semiconductors of electricity Some metalloids possess metallic luster ...
... and nonmetals on the periodic table. They have properties of both metals and nonmetals. Metalloids are more brittle than metals, less brittle than most nonmetallic solids Metalloids are semiconductors of electricity Some metalloids possess metallic luster ...
Homework Answers - Chemistry from AZ
... of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have similar chemical properties. Note there are some variations in the transition metals. Group 1 Alkali ...
... of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have similar chemical properties. Note there are some variations in the transition metals. Group 1 Alkali ...
Review sheet Atomic Structure, Electron Configuration, and Periodic
... Know that main group elements in the same group have similar properties, the same number of valence electrons and the same oxidation number. Understand that reactivity increases as you go down within a group for metals and decreases for nonmetals. Identify periods as horizontal rows on the periodic ...
... Know that main group elements in the same group have similar properties, the same number of valence electrons and the same oxidation number. Understand that reactivity increases as you go down within a group for metals and decreases for nonmetals. Identify periods as horizontal rows on the periodic ...