PowerPoint Presentation - Atoms, the Periodic Table & more review!
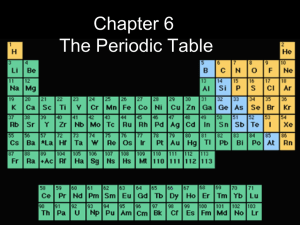
... Hydrogen does not match properties of any single group so it is placed above Group 1. • It can give it's electron away with ionic bonding, • or share it's electron in covalent bonding ...
... Hydrogen does not match properties of any single group so it is placed above Group 1. • It can give it's electron away with ionic bonding, • or share it's electron in covalent bonding ...
Periodic Trends
... • Mendeleev even went out on a limb and predicted the properties of 2 at the time undiscovered elements. • He was very accurate in his predictions, which led the world to accept his ideas about periodicity and a logical periodic table. ...
... • Mendeleev even went out on a limb and predicted the properties of 2 at the time undiscovered elements. • He was very accurate in his predictions, which led the world to accept his ideas about periodicity and a logical periodic table. ...
Practice Questions
... 2) False 3. An atom with an atomic radius smaller than that of Sulfur (S) is __________. 1) Oxygen (O) 2) Selenium (Se) 3) Calcium (Ca) 4) None of the above 4. Which two elements have the most similar chemical properties? (1) aluminum and barium (3) chlorine and sulfur (2) nickel and phosphorus (4) ...
... 2) False 3. An atom with an atomic radius smaller than that of Sulfur (S) is __________. 1) Oxygen (O) 2) Selenium (Se) 3) Calcium (Ca) 4) None of the above 4. Which two elements have the most similar chemical properties? (1) aluminum and barium (3) chlorine and sulfur (2) nickel and phosphorus (4) ...
Chapter 2:Tutorial Q: (a) What is an isotope? (b) Why are the atomic
... Solution: Atomic mass is the mass of an individual atom, whereas atomic weight is the average (weighted) of the atomic masses of an atom's naturally occurring isotopes. Q: (a) What electron subshell is being filled for the rare earth series of elements on the periodic table? (b) What electron subshe ...
... Solution: Atomic mass is the mass of an individual atom, whereas atomic weight is the average (weighted) of the atomic masses of an atom's naturally occurring isotopes. Q: (a) What electron subshell is being filled for the rare earth series of elements on the periodic table? (b) What electron subshe ...
Study Guide Chapter 6
... and explain why it is more stable than the predicted configuration. The predicted electron configuration of copper is [Ar]4s23d9. Electron promotion occurs and an electron is moved from the “s” sublevel to the “d” sublevel in order to fill the “d” sublevel. The actual electron configuration is [Ar]4 ...
... and explain why it is more stable than the predicted configuration. The predicted electron configuration of copper is [Ar]4s23d9. Electron promotion occurs and an electron is moved from the “s” sublevel to the “d” sublevel in order to fill the “d” sublevel. The actual electron configuration is [Ar]4 ...
Chapter 6 Practice Test
... Match each item with the correct statement below. a. electronegativity f. b. ionization energy g. c. atomic radius h. d. metal i. e. transition metal j. ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ...
... Match each item with the correct statement below. a. electronegativity f. b. ionization energy g. c. atomic radius h. d. metal i. e. transition metal j. ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ...
Periodic Table Student Outline
... With a publisher’s deadline looming, Mendeleyev didn’t have time to describe all 63 then-known elements. So he turned to a data set of atomic weights meticulously gathered by others. To determine those weights, scientists had passed currents through various solutions to break them up into their cons ...
... With a publisher’s deadline looming, Mendeleyev didn’t have time to describe all 63 then-known elements. So he turned to a data set of atomic weights meticulously gathered by others. To determine those weights, scientists had passed currents through various solutions to break them up into their cons ...
Chemistry 1 Chapter 4, The Periodic Table
... • group 1 – alkali metals, they have a single valence electrons and are very reactive • they are never found in nature as pure elements because they are so reactive they are always combined with other elements as compounds •group 2 – alkaline-earth metals, they have 2 valence electrons, they must lo ...
... • group 1 – alkali metals, they have a single valence electrons and are very reactive • they are never found in nature as pure elements because they are so reactive they are always combined with other elements as compounds •group 2 – alkaline-earth metals, they have 2 valence electrons, they must lo ...
D. - Taylor County Schools
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
chem 1405 chapter -5
... These are the elements of the 8A group. They have completely filled sub shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely ...
... These are the elements of the 8A group. They have completely filled sub shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely ...
Ch 6 Notes
... The B elements represent the d block; however remember to go down one for the first quantum number. The rows that are removed from the table and placed at the bottom represent the f block. Go down 2 numbers for the row number. ...
... The B elements represent the d block; however remember to go down one for the first quantum number. The rows that are removed from the table and placed at the bottom represent the f block. Go down 2 numbers for the row number. ...
Appendices: Cluster 2 Atoms and Elements
... • systematically placed the elements into an organized table. • stated that the properties of elements are a periodic function of their atomic masses and that the relationship among the elements is called periodic law. • arranged the 63 elements known at that time in order of their atomic mass so th ...
... • systematically placed the elements into an organized table. • stated that the properties of elements are a periodic function of their atomic masses and that the relationship among the elements is called periodic law. • arranged the 63 elements known at that time in order of their atomic mass so th ...
Periodic Table - Chemistry R: 4(AE)
... switch some elements out of order based on atomic mass. • In 1911, Henry Mosely, an English chemist, was examining the spectra of 38 different metals. He noticed that the wavelengths of spectra lines correlated to atomic numbers, not atomic mass. • Moseley discovered a new pattern and organized the ...
... switch some elements out of order based on atomic mass. • In 1911, Henry Mosely, an English chemist, was examining the spectra of 38 different metals. He noticed that the wavelengths of spectra lines correlated to atomic numbers, not atomic mass. • Moseley discovered a new pattern and organized the ...
CHEM121 Lecture Ch2
... Elements and Symbols • Elements: – primary substances from which all other things are built. – Cannot be broken down into simpler substances ...
... Elements and Symbols • Elements: – primary substances from which all other things are built. – Cannot be broken down into simpler substances ...
Chapter Three: Periodic Table
... Look at Group 1. The elements Li, Na, K, and Rb are listed in order of atomic number in table S. Look at how the ionization energy decreases as you go down the group. Ionization decreases as you go down a group because electrons are negative and protons are positive and the further away the electron ...
... Look at Group 1. The elements Li, Na, K, and Rb are listed in order of atomic number in table S. Look at how the ionization energy decreases as you go down the group. Ionization decreases as you go down a group because electrons are negative and protons are positive and the further away the electron ...
the modern periodic law
... The first rare gas was discovered by Ramsay around 1892, while he was studying atmospheric nitrogen. He successfully extracted argon and demonstrated that it was inert. He also discovered helium, neon, krypton, and radon. All these gases are colourless, odourless and chemically inert. For over 50 y ...
... The first rare gas was discovered by Ramsay around 1892, while he was studying atmospheric nitrogen. He successfully extracted argon and demonstrated that it was inert. He also discovered helium, neon, krypton, and radon. All these gases are colourless, odourless and chemically inert. For over 50 y ...
Unit 3 Activity Exploring Periodic Trends… MORE! Name: Directions
... Trend 1: Covalent Radius 1. Select the “Radius” bubble located towards the top of the page near the enlarged element box. 2. Click the “Covalent” bubble to the right. 3. Look at the numerical value located under each of the elements and answer the following questions: a. What does atomic covalent ra ...
... Trend 1: Covalent Radius 1. Select the “Radius” bubble located towards the top of the page near the enlarged element box. 2. Click the “Covalent” bubble to the right. 3. Look at the numerical value located under each of the elements and answer the following questions: a. What does atomic covalent ra ...
Protons, Valence Electrons, and the Periodic Table
... PROTONS Protons are the most important particle that determines the identity of the element. • The # of protons for a specific element is always the same and equals its atomic number on the periodic table ...
... PROTONS Protons are the most important particle that determines the identity of the element. • The # of protons for a specific element is always the same and equals its atomic number on the periodic table ...
Periodic Table Packet
... 19, 37,55, and 87 red, because they are wildly reactive. Color lightest at the top through darkest at the bottom to indicate the increasing reactivity of the group members. 2. Color a box around each of the alkaline earth metals, atomic, numbers 4, 12, 20, 38, 56, and 88 orange, because they are mil ...
... 19, 37,55, and 87 red, because they are wildly reactive. Color lightest at the top through darkest at the bottom to indicate the increasing reactivity of the group members. 2. Color a box around each of the alkaline earth metals, atomic, numbers 4, 12, 20, 38, 56, and 88 orange, because they are mil ...
Periodic Table and Trends Test Review KEY Describe the common
... • Most reactive metals that do not occur freely in nature • Slivery-white and softer than most other metals (to the point that they can be cut easily with a knife) • can explode if they are exposed to water 2. Alkaline earth metals (# of valence electrons = 2) • They are harder, denser, and stronger ...
... • Most reactive metals that do not occur freely in nature • Slivery-white and softer than most other metals (to the point that they can be cut easily with a knife) • can explode if they are exposed to water 2. Alkaline earth metals (# of valence electrons = 2) • They are harder, denser, and stronger ...
Document
... Al and Tl are both metals in group 3 of the periodic table, but Al ions are only ever found in the +3 state. (Al3+ cations), but Tl is known to form compounds in which there can be Tl+ or Tl3+ cations. This tendency for elements at the bottom of groups 3, 4 and 5 to form compounds in which their out ...
... Al and Tl are both metals in group 3 of the periodic table, but Al ions are only ever found in the +3 state. (Al3+ cations), but Tl is known to form compounds in which there can be Tl+ or Tl3+ cations. This tendency for elements at the bottom of groups 3, 4 and 5 to form compounds in which their out ...
7-1 Notes: Arranging the Elements
... increasing atomic _______, those that had similar properties fell into a repeating pattern. The pattern was ___________, meaning “happening at regular intervals.” His table became known as the __________ ________ of the elements. A few elements’ ____________ did not fit the pattern in Mendeleev’s ta ...
... increasing atomic _______, those that had similar properties fell into a repeating pattern. The pattern was ___________, meaning “happening at regular intervals.” His table became known as the __________ ________ of the elements. A few elements’ ____________ did not fit the pattern in Mendeleev’s ta ...
Atomic Structure and the Periodic Table of Elements: The Secret
... 1. Place 12 yellow fruit loops and 12 green fruit loops in a small mound. Take a piece of string and surround your mound. 2. On the first circle place 2 red fruit loops. Continue placing red fruit loops (total number of electrons is equal to the number of protons) onto the next energy levels until y ...
... 1. Place 12 yellow fruit loops and 12 green fruit loops in a small mound. Take a piece of string and surround your mound. 2. On the first circle place 2 red fruit loops. Continue placing red fruit loops (total number of electrons is equal to the number of protons) onto the next energy levels until y ...
Ri Christmas Lectures 2012: The Modern Alchemist
... Protons carry a relative positive charge, and have a relative mass of 1. The number of Protons in the nucleus determines which Element the atom is, and it is the number of Protons which gives an element its 'Atomic Number'. Neutrons carry no charge, and have a relative mass of 1. Neutrons make up th ...
... Protons carry a relative positive charge, and have a relative mass of 1. The number of Protons in the nucleus determines which Element the atom is, and it is the number of Protons which gives an element its 'Atomic Number'. Neutrons carry no charge, and have a relative mass of 1. Neutrons make up th ...