Periodic Table of the Elements
... – Elements in Period 1 and the first half of Period 2 gain or lose electrons in order to become like HELIUM with 2 electrons in the valence shell (Duet Rule) – Everyone else gains or loses electrons to become like Group 18 Noble Gases and have 8 electrons in the valence shell (Octet Rule) ...
... – Elements in Period 1 and the first half of Period 2 gain or lose electrons in order to become like HELIUM with 2 electrons in the valence shell (Duet Rule) – Everyone else gains or loses electrons to become like Group 18 Noble Gases and have 8 electrons in the valence shell (Octet Rule) ...
PT Trends WS
... Na, Ga, N, and F. Draw the atom (protons, neutrons, and electrons) and ion including electron configuration labeled in the energy levels. Provide the atomic symbol for all. During the formation of an ion what happens to the size of the atomic radius and ...
... Na, Ga, N, and F. Draw the atom (protons, neutrons, and electrons) and ion including electron configuration labeled in the energy levels. Provide the atomic symbol for all. During the formation of an ion what happens to the size of the atomic radius and ...
Atomic Structure and the Periodic Table
... • Top row of the two rows of metals shown outside the main body of the periodic table • “Lanthanides” (they follow lanthanum on the table) • Once believed only to be found in tiny amounts in Earth’s crust – Not as rare as originally thought – just hard to isolate in pure form Atomic Structure and th ...
... • Top row of the two rows of metals shown outside the main body of the periodic table • “Lanthanides” (they follow lanthanum on the table) • Once believed only to be found in tiny amounts in Earth’s crust – Not as rare as originally thought – just hard to isolate in pure form Atomic Structure and th ...
Lecture 10
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Z*). The follow ...
... Most electrons do not ‘feel’ the full positive charge of the nucleus. Other electrons in the atom (particularly those in lower energy orbitals) ‘shield’ some of this charge. The amount of positive charge ‘felt’ by an electron in a given orbital is called the effective nuclear charge (Z*). The follow ...
Name
... 23. Circle the letter of each statement that is true about electronegativity values. a. The electronegativity values of the transition elements are all zero. b. The element with the highest electronegativity value is sodium. c. Nonmetals have higher electronegativity values than metals. d. Electrone ...
... 23. Circle the letter of each statement that is true about electronegativity values. a. The electronegativity values of the transition elements are all zero. b. The element with the highest electronegativity value is sodium. c. Nonmetals have higher electronegativity values than metals. d. Electrone ...
KD 1
... Have them to share their information to others group. Each group have to make a resume for discussion. Give one radioactive isotope to each group and have them to make a preparation to present the use of the element in medical and industrial by making the power point slides which will be perfo ...
... Have them to share their information to others group. Each group have to make a resume for discussion. Give one radioactive isotope to each group and have them to make a preparation to present the use of the element in medical and industrial by making the power point slides which will be perfo ...
Chemistry
... - Characterized by having electrons added to the d orbital - Not as reactive as Group A elements 4. Inner Transition Metals - Elements whose outermost s sublevel and nearby f sublevel generally contain electrons - Characterized by the filling of the f orbitals - The periodic table can be divided int ...
... - Characterized by having electrons added to the d orbital - Not as reactive as Group A elements 4. Inner Transition Metals - Elements whose outermost s sublevel and nearby f sublevel generally contain electrons - Characterized by the filling of the f orbitals - The periodic table can be divided int ...
CHEM 1405 CHAPTER 5
... shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely filled (n-1) d sub shells. But are studied along with transition metals ...
... shells in the valence shell. 3. Transition Metals ( d - block elements) They are the elements of the groups IB and 3B through 8B. All of them are metals with incompletely filled (n-1) d sub shells. Elements of 2B have completely filled (n-1) d sub shells. But are studied along with transition metals ...
Homework Packet - Chemistry from AZ
... of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have similar chemical properties. Note there are some variations in the transition metals. Group 1 Alkali ...
... of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have similar chemical properties. Note there are some variations in the transition metals. Group 1 Alkali ...
Atomic Structure and the Periodic Table of Elements: The Secret
... trends or patterns found within the Periodic Table of Elements. The elements found within the Periodic Table are put in order in a very particular pattern, based on several common similarities or characteristics. In 1869, Dmitri Mendeleev produced a table of elements based on their atomic weights. P ...
... trends or patterns found within the Periodic Table of Elements. The elements found within the Periodic Table are put in order in a very particular pattern, based on several common similarities or characteristics. In 1869, Dmitri Mendeleev produced a table of elements based on their atomic weights. P ...
Ionization energy
... (Noble gases) they could not be accommodated in his table. However, the modern periodic table does draw from the concept of periods of eight. ...
... (Noble gases) they could not be accommodated in his table. However, the modern periodic table does draw from the concept of periods of eight. ...
physical science
... 2. TSW explain how Thomson and Rutherford used data from experiments to produce their atomic models. (p. 102 – 105) 3. TSW compare and contrast the three subatomic particles of an atom based on their properties. (p.108 – 110) 4. TSW distinguish the atomic number of an element from the mass number of ...
... 2. TSW explain how Thomson and Rutherford used data from experiments to produce their atomic models. (p. 102 – 105) 3. TSW compare and contrast the three subatomic particles of an atom based on their properties. (p.108 – 110) 4. TSW distinguish the atomic number of an element from the mass number of ...
5-1 Development of the Periodic Table
... • Each square represents an element. It contains its symbol, atomic number and atomic mass. The table is read like a book, from left to right, top to bottom, in order of increasing atomic number. (Protons) • http://viewpure.com/DYW50F42ss8 ...
... • Each square represents an element. It contains its symbol, atomic number and atomic mass. The table is read like a book, from left to right, top to bottom, in order of increasing atomic number. (Protons) • http://viewpure.com/DYW50F42ss8 ...
chemistry chapter 11 & 12
... – React readily with halogens to form common salts • Example: NaCl (table salt) – React readily with water to form basic solutions (alkali), hydrogen and Energy. ...
... – React readily with halogens to form common salts • Example: NaCl (table salt) – React readily with water to form basic solutions (alkali), hydrogen and Energy. ...
Lesson 4 History atom File
... 1. All matter is composed of atoms. The billiard-ball model of the atom (i.e., all atoms are tiny spheres). 2. All atoms of a given element are identical; atoms of different elements have different properties. Orally: Dalton characterized elements according to their atomic weight; however, when isot ...
... 1. All matter is composed of atoms. The billiard-ball model of the atom (i.e., all atoms are tiny spheres). 2. All atoms of a given element are identical; atoms of different elements have different properties. Orally: Dalton characterized elements according to their atomic weight; however, when isot ...
day4-periodictrends
... Do Now Work silently. Raise hand to ask Ms. Hughes anything. 1. Which family is least reactive? 2. Which family is most metallic? 3. Where are the nonmetals on the periodic table? ...
... Do Now Work silently. Raise hand to ask Ms. Hughes anything. 1. Which family is least reactive? 2. Which family is most metallic? 3. Where are the nonmetals on the periodic table? ...
An element
... • The Periodic table was designed to classify elements according to their properties and to show trends in their physical and chemical properties • The main groups are: I – Alkali metals; II – Alkaline earth metals; VII – Halogens; VIII (also called group 0) – Noble/inert gases. • The elements betw ...
... • The Periodic table was designed to classify elements according to their properties and to show trends in their physical and chemical properties • The main groups are: I – Alkali metals; II – Alkaline earth metals; VII – Halogens; VIII (also called group 0) – Noble/inert gases. • The elements betw ...
Ch 6 Notes
... 3. Mendeleev placed the known elements in a table, where he arranged elements into _______________________ with similar _____________________________. 4. Although all of the elements were not known, Mendeleev predicted the ___________________________ of several elements that were ___________________ ...
... 3. Mendeleev placed the known elements in a table, where he arranged elements into _______________________ with similar _____________________________. 4. Although all of the elements were not known, Mendeleev predicted the ___________________________ of several elements that were ___________________ ...
Word - The Chemistry Book
... A few other groups are given family names. These include the alkali metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The tr ...
... A few other groups are given family names. These include the alkali metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The tr ...
Unit Expectations – Periodic Table
... elements and/or compounds physically combined. Each element and compound has physical and chemical properties, such as boiling point, density, color, and conductivity, which are independent of the amount of the sample. 3. C4.10 A Introduced: _______ Basic: _________ Mastered: _________ I can use the ...
... elements and/or compounds physically combined. Each element and compound has physical and chemical properties, such as boiling point, density, color, and conductivity, which are independent of the amount of the sample. 3. C4.10 A Introduced: _______ Basic: _________ Mastered: _________ I can use the ...
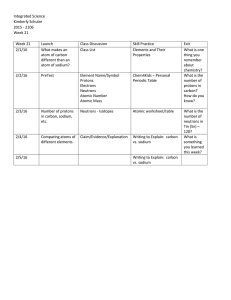
Week 21 Lessons - Highline Public Schools
... - We started the week by making observations of different pure elements. - These observations, we can see that elements are different. - In the “Support claim with evidence” section, discuss the different physical and chemical properties. - In the “Possible Scientific Explanation”, talk about the di ...
... - We started the week by making observations of different pure elements. - These observations, we can see that elements are different. - In the “Support claim with evidence” section, discuss the different physical and chemical properties. - In the “Possible Scientific Explanation”, talk about the di ...
Chemistry Unit 4: The Periodic Table – Reading
... 8. State the periodic lab. 9. What is the periodic table? 10. Where are the lanthanides and actinides located on the table, and why? Section 5.2; pp 138-149 11. Vertical columns are known as ______. What do those elements share in common? 12. Horizontal rows are known as ______. What do those elemen ...
... 8. State the periodic lab. 9. What is the periodic table? 10. Where are the lanthanides and actinides located on the table, and why? Section 5.2; pp 138-149 11. Vertical columns are known as ______. What do those elements share in common? 12. Horizontal rows are known as ______. What do those elemen ...
III. Periodic Trends
... The ease with which an atom gains an e-. For most atoms, the energy released when an e- is added. (in kJ/mol) Periodic Trend 1. Electron affinity slightly decreases down a group. 2. Electron affinity generally tends to increase across a period. ...
... The ease with which an atom gains an e-. For most atoms, the energy released when an e- is added. (in kJ/mol) Periodic Trend 1. Electron affinity slightly decreases down a group. 2. Electron affinity generally tends to increase across a period. ...