Isotopes Models
... called Deuterium. Deuterium is not radioactive. Water made from deuterium is called heavy water because the extra neutron makes it heavier. It is used in nuclear reactors. The third isotope of hydrogen is known as Tritium. It has one proton and two neutrons in its nucleus. It is radioactive. It is f ...
... called Deuterium. Deuterium is not radioactive. Water made from deuterium is called heavy water because the extra neutron makes it heavier. It is used in nuclear reactors. The third isotope of hydrogen is known as Tritium. It has one proton and two neutrons in its nucleus. It is radioactive. It is f ...
Structure - Mole Cafe
... The majority of the elements are metals. They occupy the entire left side and center of the periodic table. Nonmetals occupy the upper-right-hand ...
... The majority of the elements are metals. They occupy the entire left side and center of the periodic table. Nonmetals occupy the upper-right-hand ...
Chemistry 1 – Tollett Chapter 5 – Atomic Structure & The Periodic
... The atoms of any one element are different form those of any other element. ...
... The atoms of any one element are different form those of any other element. ...
The Atom: Idea to Theory
... – Atoms of any one element differ in properties from atoms of another element Mullis ...
... – Atoms of any one element differ in properties from atoms of another element Mullis ...
atoms - KMKunz
... When a strip of magnesium metal was burned in pure oxygen gas, 1.554 g of oxygen was consumed and the only product formed was magnesium oxide. What must have been the masses of magnesium metal burned and magnesium oxide formed? ...
... When a strip of magnesium metal was burned in pure oxygen gas, 1.554 g of oxygen was consumed and the only product formed was magnesium oxide. What must have been the masses of magnesium metal burned and magnesium oxide formed? ...
Development of the Periodic Table
... ◦ Atom: the smallest particle of an element that keeps the properties of that element. (Greek: atomos = indivisible) ...
... ◦ Atom: the smallest particle of an element that keeps the properties of that element. (Greek: atomos = indivisible) ...
Atomic Structure
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...
Chapter 2: Atoms, Molecules, and Ions
... Exam Problem. A sample of ascorbic acid (vitamin C) is synthesized in the laboratory. It contains 1.50 g of carbon and 2.00 g of oxygen. Another sample of ascorbic acid isolated from citrus fruits contains 9.22 g of carbon. How many grams of oxygen does it contain? ...
... Exam Problem. A sample of ascorbic acid (vitamin C) is synthesized in the laboratory. It contains 1.50 g of carbon and 2.00 g of oxygen. Another sample of ascorbic acid isolated from citrus fruits contains 9.22 g of carbon. How many grams of oxygen does it contain? ...
Protons
... Laura works as a consultant at a software company. The amount of her annual bonus is based upon the number of hours she works. Over summer vacation, Debbie has to read a novel for English class. She has decided to spend the same amount of time reading every day. The number of hours she spends readin ...
... Laura works as a consultant at a software company. The amount of her annual bonus is based upon the number of hours she works. Over summer vacation, Debbie has to read a novel for English class. She has decided to spend the same amount of time reading every day. The number of hours she spends readin ...
Physical Science Chapter 3 Test
... their properties will emerge in a regular pattern. 12. Because atoms of elements in the same group of the periodic table have the same number of ____________________, they have similar properties. 13. Some elements are highly ____________________ because their outermost energy levels are only partia ...
... their properties will emerge in a regular pattern. 12. Because atoms of elements in the same group of the periodic table have the same number of ____________________, they have similar properties. 13. Some elements are highly ____________________ because their outermost energy levels are only partia ...
ATOMS - Mr. Deets
... same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers Example CO2 and CO : ratio of oxygen is always 2:1 ...
... same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers Example CO2 and CO : ratio of oxygen is always 2:1 ...
Chap 03A-Atoms and Elements.pptx
... Ø explains the difference between an element and a compound. Ø explains two scientific laws, and Ø predicts a new scientific law. ...
... Ø explains the difference between an element and a compound. Ø explains two scientific laws, and Ø predicts a new scientific law. ...
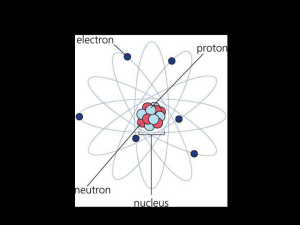
The atomic number tells how many protons Protons make an atom
... Atoms of the same element with different atomic masses are called isotopes Gold has a mass of 196.97 That means MOST gold atoms have 197 p+ and no, but some rare atoms will have only 196. They ALL have 79 p+. Most have 118no, but a few may have 117 no. ...
... Atoms of the same element with different atomic masses are called isotopes Gold has a mass of 196.97 That means MOST gold atoms have 197 p+ and no, but some rare atoms will have only 196. They ALL have 79 p+. Most have 118no, but a few may have 117 no. ...
Electrons and the Atom PPT
... a shell contains the maximum number of electrons, it is said to be filled. Electrons in the outer shell of an atom are known as valence electrons and the shell is the valence shell. The valence electrons are the only electrons involved in forming chemical bonds ...
... a shell contains the maximum number of electrons, it is said to be filled. Electrons in the outer shell of an atom are known as valence electrons and the shell is the valence shell. The valence electrons are the only electrons involved in forming chemical bonds ...
Thinking about Atomic Mass and Density sheet
... Every element has a different number of protons. Scientists have given each element a number based on the number of protons in an atom of that element. This number is called an atomic number. Each element’s atomic number is unique. The higher the atomic number, the more protons an element has. So fo ...
... Every element has a different number of protons. Scientists have given each element a number based on the number of protons in an atom of that element. This number is called an atomic number. Each element’s atomic number is unique. The higher the atomic number, the more protons an element has. So fo ...
Learning About The Atom and Atomic Structure
... tube and the deflections caused by negatively charged particles) From experiments, Thomson determined the charge to mass ratio of an electron (e/m = -1.76 x 108 C/g, where C is in Coulombs and represents charge, and m represents the electron mass in grams) (If students ask: it is not required to m ...
... tube and the deflections caused by negatively charged particles) From experiments, Thomson determined the charge to mass ratio of an electron (e/m = -1.76 x 108 C/g, where C is in Coulombs and represents charge, and m represents the electron mass in grams) (If students ask: it is not required to m ...
Finding the Amounts of Subatomic Particles
... properties as the element. The nucleus is the central part of an atom. It is made up of protons and neutrons and contains most of the atom’s mass. The nucleus was discovered by Ernest Rutherford in 1911. ...
... properties as the element. The nucleus is the central part of an atom. It is made up of protons and neutrons and contains most of the atom’s mass. The nucleus was discovered by Ernest Rutherford in 1911. ...
1030133Notes 4.3
... Finding a weighted average atomic mass (% abundance isotope 1 x mass isotope 1 ) + (% abundance isotope 2 x mass isotope 2) + (% abundance isotope 3 x mass isotope 3) + ……..Continue for all the isotopes you have ...
... Finding a weighted average atomic mass (% abundance isotope 1 x mass isotope 1 ) + (% abundance isotope 2 x mass isotope 2) + (% abundance isotope 3 x mass isotope 3) + ……..Continue for all the isotopes you have ...
Reporting Category 2: Atomic Structure and Nuclear Chemistry
... The energy levels of electrons are labeled by principal quantum numbers (n). These numbers have positive integer values of 1, 2, 3, and so on. Electrons with the same principal quantum number are in the same principal energy level. Each level contains one or more sublevels. Each sublevel contains on ...
... The energy levels of electrons are labeled by principal quantum numbers (n). These numbers have positive integer values of 1, 2, 3, and so on. Electrons with the same principal quantum number are in the same principal energy level. Each level contains one or more sublevels. Each sublevel contains on ...
The nucleus Rutherford`s nuclear atom (1902
... • Proposed the existence of isotopes – In the early 1900s, scientists discovered dozens of 'new' radioactive elements which could not be fitted into the ten or so gaps in the Periodic Table. – However, it was found that some of these elements had identical chemical properties although their radioact ...
... • Proposed the existence of isotopes – In the early 1900s, scientists discovered dozens of 'new' radioactive elements which could not be fitted into the ten or so gaps in the Periodic Table. – However, it was found that some of these elements had identical chemical properties although their radioact ...
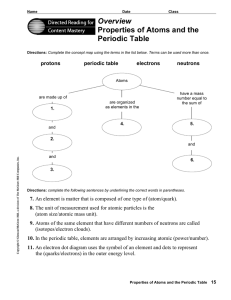
Reading Assignment Worksheet on Atoms - District 196 e
... Directions: Complete the concept map using the terms in the list below. Terms can be used more than once. ...
... Directions: Complete the concept map using the terms in the list below. Terms can be used more than once. ...
Chem Ch4,25
... rather than a bunch of grapes. Some isotopes of some elements contain and unstable ratio of protons to neutrons. These Radioisotopes are radioactive because they have unstable nuclei. ...
... rather than a bunch of grapes. Some isotopes of some elements contain and unstable ratio of protons to neutrons. These Radioisotopes are radioactive because they have unstable nuclei. ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.