Chem Ch4,25
... rather than a bunch of grapes. Some isotopes of some elements contain and unstable ratio of protons to neutrons. These Radioisotopes are radioactive because they have unstable nuclei. ...
... rather than a bunch of grapes. Some isotopes of some elements contain and unstable ratio of protons to neutrons. These Radioisotopes are radioactive because they have unstable nuclei. ...
Unit 3 Rev Pckt - Old Saybrook Public Schools
... a. the nucleus of an atom occupies most of an atom's volume. b. positive charges are dispersed throughout the atom. c. positive charges are concentrated in a very small core at the atom's center. d. protons and neutrons are located in the nucleus. 23. Scientists have determined that electrons a. mov ...
... a. the nucleus of an atom occupies most of an atom's volume. b. positive charges are dispersed throughout the atom. c. positive charges are concentrated in a very small core at the atom's center. d. protons and neutrons are located in the nucleus. 23. Scientists have determined that electrons a. mov ...
Nuclear Chemistry - Solon City Schools
... 2. List the subatomic parts of the atom including quarks 3. Explain the difference in isotopes of an atom 4. Explain alpha, beta, and gamma radiation and what happens to the nucleus 5. Calculate half-lives of radioactive materials 6. List differences between a fusion and fission reactions ...
... 2. List the subatomic parts of the atom including quarks 3. Explain the difference in isotopes of an atom 4. Explain alpha, beta, and gamma radiation and what happens to the nucleus 5. Calculate half-lives of radioactive materials 6. List differences between a fusion and fission reactions ...
Unit 2- The Atom
... on the blank side. Record the number of “S.” To flip the skittles, shake the bag containing the skittles and pour the contents out on the paper towel. The skittles that have landed on the blank side have decayed and may be consumed. 2. Flip the skittles that landed on the “S” and separate as ...
... on the blank side. Record the number of “S.” To flip the skittles, shake the bag containing the skittles and pour the contents out on the paper towel. The skittles that have landed on the blank side have decayed and may be consumed. 2. Flip the skittles that landed on the “S” and separate as ...
Atomic Structure
... Masses of subatomic particles ridiculously small – needed something more convenient Arbitrary amount set: Carbon-12 atom = 12.00000 amu 1 amu (atomic mass unit) = 1/12 mass of C-12 atom Mass of a single proton/neutron – 1 amu Why are the masses decimals? Most elements exist as a mixture of 2 or mo ...
... Masses of subatomic particles ridiculously small – needed something more convenient Arbitrary amount set: Carbon-12 atom = 12.00000 amu 1 amu (atomic mass unit) = 1/12 mass of C-12 atom Mass of a single proton/neutron – 1 amu Why are the masses decimals? Most elements exist as a mixture of 2 or mo ...
The Periodic Table HL Page 1 of 3 G. Galvin Name: Periodic Table
... -list the numbers of electrons in each main energy level in atoms of numbers 1-20 -build up the electronic structure of the first 36 elements -derive the electronic configurations of ions of s- and p-block elements only -describe the arrangement of electrons in individual orbitals of p-block atom ...
... -list the numbers of electrons in each main energy level in atoms of numbers 1-20 -build up the electronic structure of the first 36 elements -derive the electronic configurations of ions of s- and p-block elements only -describe the arrangement of electrons in individual orbitals of p-block atom ...
The Modern Atomic Model
... • Every atom of an element will always have the same number of protons • Carbon will always have 6 protons, oxygen will always have 8 protons, and iron will always have 26 protons. ...
... • Every atom of an element will always have the same number of protons • Carbon will always have 6 protons, oxygen will always have 8 protons, and iron will always have 26 protons. ...
Elements, Ions and Isotopes
... (Isotope 1 Mass x Isotope 1 Abundance) + (Isotope 2 Mass x Isotope 2 Abundance) … • 6Li = 7.5% abundant and 7Li = 92.5% –Avg. Atomic mass of Li = ______________ ...
... (Isotope 1 Mass x Isotope 1 Abundance) + (Isotope 2 Mass x Isotope 2 Abundance) … • 6Li = 7.5% abundant and 7Li = 92.5% –Avg. Atomic mass of Li = ______________ ...
Structure of the Atom
... 3) Atoms combine in whole-number ratios to form compounds 4) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element. ...
... 3) Atoms combine in whole-number ratios to form compounds 4) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element. ...
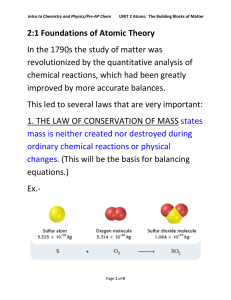
2:1 Foundations of Atomic Theory In the 1790s the study of matter
... are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This means that in our current model of the atom ...
... are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This means that in our current model of the atom ...
class slides for Chapter 42
... 42.3: Some Nuclear Properties: Nuclei are made up of protons and neutrons. The number of protons in a nucleus is called the atomic number of the nucleus, and is represented by the symbol Z; the number of neutrons is the neutron number, and is represented by the symbol N. The total number of neutron ...
... 42.3: Some Nuclear Properties: Nuclei are made up of protons and neutrons. The number of protons in a nucleus is called the atomic number of the nucleus, and is represented by the symbol Z; the number of neutrons is the neutron number, and is represented by the symbol N. The total number of neutron ...
Test #1 Study Guide
... Robert Millikan – Through the Oil Drop experiment, deduced that the mass of an electron was about 200 times lighter than a hydrogen atom. Ernest Rutherford – Through his gold foil experiment in which he shot particles through a piece of gold foil and recorded where these particles ended up, he dis ...
... Robert Millikan – Through the Oil Drop experiment, deduced that the mass of an electron was about 200 times lighter than a hydrogen atom. Ernest Rutherford – Through his gold foil experiment in which he shot particles through a piece of gold foil and recorded where these particles ended up, he dis ...
Atomic Structure - Coronado High School
... Atoms of the same element are identical. Atoms of any one element are different from those of any other elements. Atoms of different elements can physically mix together or can chemically combine with another in simple or whole number ratios to form compounds. Chemical reactions occur when ato ...
... Atoms of the same element are identical. Atoms of any one element are different from those of any other elements. Atoms of different elements can physically mix together or can chemically combine with another in simple or whole number ratios to form compounds. Chemical reactions occur when ato ...
worksheet #1 - chemistryrocks.net
... tables are “weighted averages” of the weights of the different naturally occurring isotopes of the element. Let’s look at an example. Approximately 75% of the chlorine atoms found in nature have a mass of 35. The other 25% have a mass of 37. What should we report as the atomic weight for chlorine? W ...
... tables are “weighted averages” of the weights of the different naturally occurring isotopes of the element. Let’s look at an example. Approximately 75% of the chlorine atoms found in nature have a mass of 35. The other 25% have a mass of 37. What should we report as the atomic weight for chlorine? W ...
Name Period _____ Chemistry Review
... ____ 13. A change that produces one or more new substances is called a physical change. _________________________ ____ 14. A(n) pure substance is made of only one kind of matter and has definite properties. _________________________ ____ 15. A substance that undergoes a chemical change is still the ...
... ____ 13. A change that produces one or more new substances is called a physical change. _________________________ ____ 14. A(n) pure substance is made of only one kind of matter and has definite properties. _________________________ ____ 15. A substance that undergoes a chemical change is still the ...
Chemistry 515 Name: L. S. Curtin Soc. Sec. #: February 8, 1999
... 11) Which of the following statements about Daltons Atomic Theory has been shown to be incorrect? a) b) c) d) e) ...
... 11) Which of the following statements about Daltons Atomic Theory has been shown to be incorrect? a) b) c) d) e) ...
Unit 3 Power Point
... What is a compound? Compoundchemical combination of elements. Each has its own unique and identifiable characteristics. Cannot be separated by physical means. Can be separated by chemical means. Example: Na is explosive when wet. Cl2 is a poisonous gas. When combined, they produce the compound know ...
... What is a compound? Compoundchemical combination of elements. Each has its own unique and identifiable characteristics. Cannot be separated by physical means. Can be separated by chemical means. Example: Na is explosive when wet. Cl2 is a poisonous gas. When combined, they produce the compound know ...
Document
... or 3He and 4He, are the two isotopes of helium with mass numbers of 3 and 4, with 1 and 2 neutrons respectively but both have 2 protons. Helium-3 is formed in the Sun by the initial nuclear fusion process. Helium-4 is also formed in the Sun and as a product of radioactive alpha decay of an unstable ...
... or 3He and 4He, are the two isotopes of helium with mass numbers of 3 and 4, with 1 and 2 neutrons respectively but both have 2 protons. Helium-3 is formed in the Sun by the initial nuclear fusion process. Helium-4 is also formed in the Sun and as a product of radioactive alpha decay of an unstable ...
Unit #3 Atoms / Atomic Structure / Subatomic Particles
... What is a compound? Compoundchemical combination of elements. Each has its own unique and identifiable characteristics. Cannot be separated by physical means. Can be separated by chemical means. Example: Na is explosive when wet. Cl2 is a poisonous gas. When combined, they produce the compound know ...
... What is a compound? Compoundchemical combination of elements. Each has its own unique and identifiable characteristics. Cannot be separated by physical means. Can be separated by chemical means. Example: Na is explosive when wet. Cl2 is a poisonous gas. When combined, they produce the compound know ...
Drawing Atomic Structure
... An atom is the smallest particle of an element that still retains the properties of that element. Atomic Symbol - The shorthand abbreviation that is used to identify an element (element name) - Atomic symbols have o A capital letter at the beginning of the symbol (all symbols have this) o Some have ...
... An atom is the smallest particle of an element that still retains the properties of that element. Atomic Symbol - The shorthand abbreviation that is used to identify an element (element name) - Atomic symbols have o A capital letter at the beginning of the symbol (all symbols have this) o Some have ...
Atomic Masses Notes
... of the same element that have different numbers of neutrons are called isotopes. ...
... of the same element that have different numbers of neutrons are called isotopes. ...
Chapter 4 Notes
... He determined the actual charge of an electron. His experimental setup and technique was so good that the charge he measured almost 100 years ago is within 1% of the currently accepted value. ...
... He determined the actual charge of an electron. His experimental setup and technique was so good that the charge he measured almost 100 years ago is within 1% of the currently accepted value. ...
1 Subatomic Particles – Lets Review Again! General Information: An
... 1. There are two naturally occurring isotopes of boron. Their atomic masses are 10B (10.0129 amu) and 11B (11.00931 amu). Without using a calculator, choose the best estimate among the following for the percent abundances for the two isotopes. (Hint: Refer to the periodic table for the average atomi ...
... 1. There are two naturally occurring isotopes of boron. Their atomic masses are 10B (10.0129 amu) and 11B (11.00931 amu). Without using a calculator, choose the best estimate among the following for the percent abundances for the two isotopes. (Hint: Refer to the periodic table for the average atomi ...
Atoms = basic unit of matter
... abundance of each isotope of a particular atom. Atomic mass unit (amu) is a unit of mass equal to 1/12th the mass of a carbon-12 atom. amu’s are used instead of grams because the masses of subatomic particles are small More useful to compare the relative masses of atoms using a reference isoto ...
... abundance of each isotope of a particular atom. Atomic mass unit (amu) is a unit of mass equal to 1/12th the mass of a carbon-12 atom. amu’s are used instead of grams because the masses of subatomic particles are small More useful to compare the relative masses of atoms using a reference isoto ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.