05: Atoms and Molecules
... • Neutrons do not affect charge. • Neutrons do affect mass (neutron mass = 1 amu). • Isotopes of the same element will have different masses. • Masses are shown in the upper right corner of the symbol or after the elements name: e.g.: 13C or Carbon-13 Mass Number # of protons + # of neutrons Always ...
... • Neutrons do not affect charge. • Neutrons do affect mass (neutron mass = 1 amu). • Isotopes of the same element will have different masses. • Masses are shown in the upper right corner of the symbol or after the elements name: e.g.: 13C or Carbon-13 Mass Number # of protons + # of neutrons Always ...
File - Ms. Gutierrez`s Chemistry Website
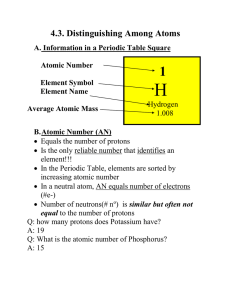
... Scientist name Henry Moseley • Atomic Number is the number of protons in the nucleus of an atom. • The atoms of each element differ from the atom of the element before it by one proton. • In atoms that are electrically neutral, atomic number also is the number of electrons. ...
... Scientist name Henry Moseley • Atomic Number is the number of protons in the nucleus of an atom. • The atoms of each element differ from the atom of the element before it by one proton. • In atoms that are electrically neutral, atomic number also is the number of electrons. ...
Chapter 3 Powerpoint
... 3. Atoms of different elements have different properties. 4. Atoms of different elements combine in simple whole-number ...
... 3. Atoms of different elements have different properties. 4. Atoms of different elements combine in simple whole-number ...
File
... Station 5: Cl-Ev-R summary of Beanium Use the following format to create a lab summary report based on your Beanium Lab : How do the different beanium isotopes represent an isotope in real life ? Start your Claim based on what you know about Isotopes. This Report is due on Friday September 13, 2013 ...
... Station 5: Cl-Ev-R summary of Beanium Use the following format to create a lab summary report based on your Beanium Lab : How do the different beanium isotopes represent an isotope in real life ? Start your Claim based on what you know about Isotopes. This Report is due on Friday September 13, 2013 ...
partsofatom
... center of an atom Small, dense (packed tightly) Contains protons and neutrons: most of the atom’s mass So dense, if it were the size of a grape, it would weigh over 9 million ...
... center of an atom Small, dense (packed tightly) Contains protons and neutrons: most of the atom’s mass So dense, if it were the size of a grape, it would weigh over 9 million ...
Notes 4.3 filled in
... each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exists as multiple versions, called isotopes Isotopes of the same element are identica ...
... each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exists as multiple versions, called isotopes Isotopes of the same element are identica ...
Properties of Atoms and the Periodic Table
... 1. amu appropriate unit for measuring the size of a particle 2. equals one-twelfth the mass of a carbon atom C. Protons 1. number of protons is used to identify elements 2. each element has different number of protons 3. number of electrons equals the number of protons in an element ...
... 1. amu appropriate unit for measuring the size of a particle 2. equals one-twelfth the mass of a carbon atom C. Protons 1. number of protons is used to identify elements 2. each element has different number of protons 3. number of electrons equals the number of protons in an element ...
Carbon Isotopes
... condition which was almost certainly caused by working with pitchblende. The medical condition was aplastic anaemia which is known to be caused by exposure to radiation. ...
... condition which was almost certainly caused by working with pitchblende. The medical condition was aplastic anaemia which is known to be caused by exposure to radiation. ...
Matter Unit Study Guide Phases of Matter
... What is the mass of the object on this triple beam balance? ...
... What is the mass of the object on this triple beam balance? ...
CHM 103 Lecture 5 S07
... same as stable isotopes of the same atom Thus, can use to target an organ or a physiological process Usually -emitters because radiation has to emerge from the body if the imaging equipment is to see it ...
... same as stable isotopes of the same atom Thus, can use to target an organ or a physiological process Usually -emitters because radiation has to emerge from the body if the imaging equipment is to see it ...
Isotopes
... Isotopes are atoms of the same chemical element, each having a different mass number (different number of neutrons). Isotopes differ in mass number but never in atomic number (# of protons). Since we cannot see atoms, you will use M&M’s to represent atoms. The purpose of this lab is to calculate the ...
... Isotopes are atoms of the same chemical element, each having a different mass number (different number of neutrons). Isotopes differ in mass number but never in atomic number (# of protons). Since we cannot see atoms, you will use M&M’s to represent atoms. The purpose of this lab is to calculate the ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Atoms are made mostly out of (+) charged material, like dough in a bun. The (-) charged electrons are found inside the (+) dough. ...
... Atoms are made mostly out of (+) charged material, like dough in a bun. The (-) charged electrons are found inside the (+) dough. ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Atoms are made mostly out of (+) charged material, like dough in a bun. The (-) charged electrons are found inside the (+) dough. ...
... Atoms are made mostly out of (+) charged material, like dough in a bun. The (-) charged electrons are found inside the (+) dough. ...
Nothing exists except atoms and empty space
... a question, your answer must be a correctly numbered restatement of the question or statement followed by a series of complete sentences. No phrases, partial answers or isolated numbers will be given credit! Your answer must stand alone without the reader knowing the question asked or statement made ...
... a question, your answer must be a correctly numbered restatement of the question or statement followed by a series of complete sentences. No phrases, partial answers or isolated numbers will be given credit! Your answer must stand alone without the reader knowing the question asked or statement made ...
Atoms - SD308.org
... He never developed a theory because he did not have experimental support nor did he explain chemical behavior. It took 2000 years after Democritus for the real nature of atoms and events at the atomic level to be established ...
... He never developed a theory because he did not have experimental support nor did he explain chemical behavior. It took 2000 years after Democritus for the real nature of atoms and events at the atomic level to be established ...
atomic number
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Atomic Structure
... Atoms of the same element are identical. The atoms of any one element are different from those of any other element. Atoms of different elements can chemically combine in simple whole-number ratios to form compounds. Atoms of one element are never changed into atoms of another element in a ...
... Atoms of the same element are identical. The atoms of any one element are different from those of any other element. Atoms of different elements can chemically combine in simple whole-number ratios to form compounds. Atoms of one element are never changed into atoms of another element in a ...
Atomic Structure
... showed that the majority of an atom is empty Microscope (STM) space, with most of its mass concentrated in a tiny nucleus. ...
... showed that the majority of an atom is empty Microscope (STM) space, with most of its mass concentrated in a tiny nucleus. ...
Worksheet - Chapter 3A - Atomic Structure 2012 Atomic Theory
... showed that the majority of an atom is empty Microscope (STM) space, with most of its mass concentrated in a tiny nucleus. ...
... showed that the majority of an atom is empty Microscope (STM) space, with most of its mass concentrated in a tiny nucleus. ...
9April2012 Notes
... atomic # from it. (11) A mole is a unit of quantity. It is equal to Avogadro’s Number which is 6.022 x 1023 atoms or molecules or particles. Because each element has a different # of protons & neutrons, each will have a different molar mass (another name for the “weighted average mass”). For instanc ...
... atomic # from it. (11) A mole is a unit of quantity. It is equal to Avogadro’s Number which is 6.022 x 1023 atoms or molecules or particles. Because each element has a different # of protons & neutrons, each will have a different molar mass (another name for the “weighted average mass”). For instanc ...
The diameter of a Ni atom is
... formed when atoms with specific weight ratio. different atomic numbers combine in a specific ratio • Element: pure substance • Element: substance whose that cannot be decomposed or atoms all have the same converted to a simpler atomic number (number of substance. protons). • Both are characterized b ...
... formed when atoms with specific weight ratio. different atomic numbers combine in a specific ratio • Element: pure substance • Element: substance whose that cannot be decomposed or atoms all have the same converted to a simpler atomic number (number of substance. protons). • Both are characterized b ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.