atomic number - geraldinescience
... • Based on similarities in their chemical properties, elements on the periodic table are arranged in columns, which are called groups. • An atom’s chemical properties are largely determined by the number of the outermost electrons in an atom’s electron cloud. These electrons are called valence elect ...
... • Based on similarities in their chemical properties, elements on the periodic table are arranged in columns, which are called groups. • An atom’s chemical properties are largely determined by the number of the outermost electrons in an atom’s electron cloud. These electrons are called valence elect ...
Atoms/Atomic Theory PPT
... Thomson (English 1897) did more experiments to actually make the discovery he found ratio of charge of this particle to this mass of the particle since the ratio stayed constant for any metal that contained it, it must be the same in all of the metals ...
... Thomson (English 1897) did more experiments to actually make the discovery he found ratio of charge of this particle to this mass of the particle since the ratio stayed constant for any metal that contained it, it must be the same in all of the metals ...
Chapter 5 Atomic Structure and Periodic Table 2014
... atoms of any one element are different from those of any other atom. ...
... atoms of any one element are different from those of any other atom. ...
Understanding the Atom
... covered a photographic plate with black paper, this energy would pass through the paper and expose the film. An image of the mineral appeared on the plate. One day, Becquerel left the mineral in a drawer next to a wrapped, unexposed plate. Later, he unwrapped the plate and found that it contained an ...
... covered a photographic plate with black paper, this energy would pass through the paper and expose the film. An image of the mineral appeared on the plate. One day, Becquerel left the mineral in a drawer next to a wrapped, unexposed plate. Later, he unwrapped the plate and found that it contained an ...
Intro to Atoms
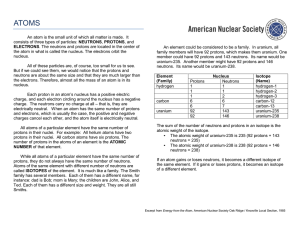
... atoms that have more particles (neutrons) in it’s nucleus. For example, you know that Hydrogen has only 1 proton, so it has an atomic number of 1. However, if you add neutrons to it’s nucleus, it will still have an atomic number of 1, but the atomic mass has changed. This “heavier” atom is called an ...
... atoms that have more particles (neutrons) in it’s nucleus. For example, you know that Hydrogen has only 1 proton, so it has an atomic number of 1. However, if you add neutrons to it’s nucleus, it will still have an atomic number of 1, but the atomic mass has changed. This “heavier” atom is called an ...
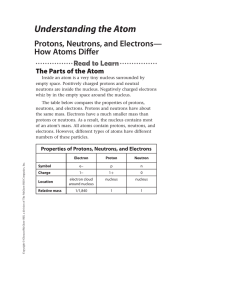
An atom is the small unit of which all matter is made. It consists of
... ATOMS An atom is the small unit of which all matter is made. It consists of three types of particles: NEUTRONS, PROTONS, and ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course ...
... ATOMS An atom is the small unit of which all matter is made. It consists of three types of particles: NEUTRONS, PROTONS, and ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course ...
Homework #1 Atoms
... ____________ of that element. Because atoms are electrically neutral, the number of protons and ____________ in an atom are equal. 2. The sum of the _____________ and neutrons is the mass number. 3. Atoms of the same element are identical in most respects, but they can differ in the number of ______ ...
... ____________ of that element. Because atoms are electrically neutral, the number of protons and ____________ in an atom are equal. 2. The sum of the _____________ and neutrons is the mass number. 3. Atoms of the same element are identical in most respects, but they can differ in the number of ______ ...
Document
... What happens when an element emits radioactive particles? A. It gains energy. B. It gains neutrons. ...
... What happens when an element emits radioactive particles? A. It gains energy. B. It gains neutrons. ...
Topic 1 - Periodic Table
... Loss of electrons from neutral atoms results in the formation of an ion with a positive charge (cation). Gain of electrons by a neutral atom results in the formation of an ion with a negative charge (anion). Transition metals can have multiple oxidation states. Matter occurs as elements (pure), comp ...
... Loss of electrons from neutral atoms results in the formation of an ion with a positive charge (cation). Gain of electrons by a neutral atom results in the formation of an ion with a negative charge (anion). Transition metals can have multiple oxidation states. Matter occurs as elements (pure), comp ...
Subatomic Particles - Ciencias Esmeralda
... Thomson (1912) found 2 types of neon atoms and Soddy (1910) found 2 types of uranium atoms. 2 elements that have the same atomic number but different mass numbers Based on atomic structure: 2 elements that have the same number of protons but different number of neutrons. For example: Cl-35 and Cl-37 ...
... Thomson (1912) found 2 types of neon atoms and Soddy (1910) found 2 types of uranium atoms. 2 elements that have the same atomic number but different mass numbers Based on atomic structure: 2 elements that have the same number of protons but different number of neutrons. For example: Cl-35 and Cl-37 ...
Chemistry Atoms Learning Objectives Atoms Essential knowledge
... in the order of 10-15 m. We now know that they are not the fundamental particles. Take, for example, a proton. It is now believed a proton is made of three fundamental particles called quarks. There are six different types of quarks, of which only up (u) quark and down (d) quark made up of protons: ...
... in the order of 10-15 m. We now know that they are not the fundamental particles. Take, for example, a proton. It is now believed a proton is made of three fundamental particles called quarks. There are six different types of quarks, of which only up (u) quark and down (d) quark made up of protons: ...
Explaining the Periodic Table (6.7)
... • If elements are the building blocks of all other matter, what are they made of? • There are three particles that make up an atom or element: • protons • electrons • neutrons • These are called subatomic particles because they are smaller or below an atom. ...
... • If elements are the building blocks of all other matter, what are they made of? • There are three particles that make up an atom or element: • protons • electrons • neutrons • These are called subatomic particles because they are smaller or below an atom. ...
File
... A fourth part, the nucleus, is often referred to but we are not focusing on it as much as we are on protons, neutrons, and electrons. The nucleus is the central part of the atom. Protons and neutrons are located in the nucleus or central part of an atom. Electrons constantly orbit around the nucleus ...
... A fourth part, the nucleus, is often referred to but we are not focusing on it as much as we are on protons, neutrons, and electrons. The nucleus is the central part of the atom. Protons and neutrons are located in the nucleus or central part of an atom. Electrons constantly orbit around the nucleus ...
PPT_Topic2
... number of an element and explain what they mean, and state that electrons are found in shells. We will do this by Analysing nuclide notation and drawing diagrams to show electron structure. We will have succeeded if We can draw a diagram and explain important atom numbers to another pupil. ...
... number of an element and explain what they mean, and state that electrons are found in shells. We will do this by Analysing nuclide notation and drawing diagrams to show electron structure. We will have succeeded if We can draw a diagram and explain important atom numbers to another pupil. ...
Atomic Structure
... How would the results of Rutherford's experiments have been different if atoms had the positive charges on the outside and negative charges in the middle? ...
... How would the results of Rutherford's experiments have been different if atoms had the positive charges on the outside and negative charges in the middle? ...
Problem Set 4
... how they change, what changes about them and how humans have tried to understand and organize nature. 29) Why do you think we are starting with the history of the atom? Again answers will vary, but the historical evidence allows you a perspective of how humans have improved what we understand to be ...
... how they change, what changes about them and how humans have tried to understand and organize nature. 29) Why do you think we are starting with the history of the atom? Again answers will vary, but the historical evidence allows you a perspective of how humans have improved what we understand to be ...
Atoms
... Key Facts about Isotopes • Isotopes: – Atoms of the same element – BUT, Have different numbers of neutrons • Atomic number on periodic table does not change (Same # of Protons) • Atomic Mass (amu) on periodic table does not change (Average of most common isotopes) • Mass number changes (Actual numb ...
... Key Facts about Isotopes • Isotopes: – Atoms of the same element – BUT, Have different numbers of neutrons • Atomic number on periodic table does not change (Same # of Protons) • Atomic Mass (amu) on periodic table does not change (Average of most common isotopes) • Mass number changes (Actual numb ...
Ions - amyschaefer24
... element but have different masses. •Atoms that have the same number of protons but a different number of neutrons. •Why doesn’t an isotope form if we change the number of protons? ...
... element but have different masses. •Atoms that have the same number of protons but a different number of neutrons. •Why doesn’t an isotope form if we change the number of protons? ...
Atomic Number
... • Use the atomic number given to find the element. • Write the element symbol on the blanks (1st letter of the symbol is capitalized, 2nd (if present) is lower case. • Then write the full name of each element. • Periodic tables can be found in your agenda book or in the textbook inside covers. ...
... • Use the atomic number given to find the element. • Write the element symbol on the blanks (1st letter of the symbol is capitalized, 2nd (if present) is lower case. • Then write the full name of each element. • Periodic tables can be found in your agenda book or in the textbook inside covers. ...
Chapter 17 - murraysphysical
... These are the actual wood spheres that Dalton used as models for atoms. They are about 200 years old. Notice the holes drilled in them. He probably used them to connect atoms to other atoms to make compounds. ...
... These are the actual wood spheres that Dalton used as models for atoms. They are about 200 years old. Notice the holes drilled in them. He probably used them to connect atoms to other atoms to make compounds. ...
Yr11 Chemistry Title Page:TourismContents
... Atoms of different elements can combine with one another in simple (whole number) ratios to form compounds. ...
... Atoms of different elements can combine with one another in simple (whole number) ratios to form compounds. ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.